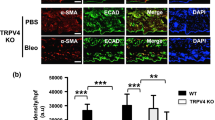
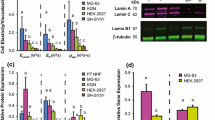
Abstract
Long non-coding RNAs (LncRNAs) are long (> 200 bases), non-coding, single-stranded RNAs that have emerged as major regulators of gene expression, cell differentiation, development, and oncogenesis. In view of the fact that matrix stiffness plays a role in cellular functions associated with these processes, it is important to ask what role matrix stiffness plays in regulating expression of LncRNAs. In this report, we show that (i) matrix stiffness causes differential expression of epithelial–mesenchymal transition (EMT)-related LncRNAs and mRNAs in primary mouse normal epidermal keratinocytes, (ii) differential expression of EMT-related LncRNAs and mRNAs occurs in response to combined stimulation of transforming growth factor β1 and matrix stiffness, and (iii) transient receptor potential (TRP) channel of the vanilloid subfamily, TRPV4, a matrix stiffness-sensitive ion channel, plays a role in differential expression of EMT-related LncRNAs and mRNAs in response to combined stimulation by TGFβ1 and matrix stiffness. These data identify TRPV4 as a candidate plasma membrane mechanosensor that transmits matrix-sensing signals essential to LncRNA expression. Our results also show that we have established and validated an assay system capable of discovering novel LncRNAs and mRNAs sensitive to matrix stiffening.




Similar content being viewed by others
Data availability
All data are freely available from the corresponding author as per University of Maryland policy of availability of scientific data.
References
Gepner AD, Korcarz CE, Colangelo LA, Hom EK, Tattersall MC, Astor BC, Kaufman JD, Liu K, Stein JH (2014) Longitudinal effects of a decade of aging on carotid artery stiffness: the multiethnic study of atherosclerosis. Stroke 45(1):48–53. https://doi.org/10.1161/STROKEAHA.113.002649
van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, Hoeks AP, van der Kuip DA, Hofman A, Witteman JC (2001) Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke 32(2):454–460. https://doi.org/10.1161/01.str.32.2.454
Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A (2001) Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 37(5):1236–1241. https://doi.org/10.1161/01.hyp.37.5.1236
Mitchell GF, Hwang S-J, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ (2010) Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 21(4):505–511. https://doi.org/10.1161/CIRCULATIONAHA.109.886655
Lamouille S, Xu J, Derynck R (2014) Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol 15(3):178–196. https://doi.org/10.1038/nrm3758
Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA (2009) Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 119(6):1438–1449. https://doi.org/10.1172/JCI38019
Chapman HA (2011) Epithelial-mesenchymal interactions in pulmonary fibrosis. Annu Rev Physiol 73:413–435. https://doi.org/10.1146/annurev-physiol-012110-142225
Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119:1420–1428. https://doi.org/10.1172/JCI39104
Gjorevski N, Boghaert E, Nelson CM (2012) Regulation of epithelial-mesenchymal transition by transmission of mechanical stress through epithelial tissues. Cancer Microenviron 5(1):29–38. https://doi.org/10.1007/s12307-011-0076-5
Moustakas A, Heldin CH (2016) Mechanisms of TGFβ-induced epithelial-mesenchymal transition. J Clin Med 5(7):63. https://doi.org/10.3390/jcm5070063
Leight JL, Wozniak MA, Chen S, Lynch ML, Chen CS (2012) Matrix rigidity regulates a switch between TGF-β1–induced apoptosis and epithelial-mesenchymal transition. Mol Biol Cell 23(5):781–791. https://doi.org/10.1091/mbc.E11-06-0537
Wei SC, Fattet L, Tsai JH, Guo Y, Pai VH, Majeski HE, Chen AC, Sah RL, Taylor SS, Engler AJ, Yang J (2015) Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat Cell Biol 17(5):678–688. https://doi.org/10.1038/ncb3157
Scheraga RG, Abraham S, Niese KA, Southern BD, Grove LM, Hite RD, McDonald C, Hamilton TA, Olman MA (2016) TRPV4 mechanosensitive ion channel regulates lipopolysaccharide-stimulated macrophage phagocytosis. J Immunol 196(1):428–436. https://doi.org/10.4049/jimmunol.1501688
Rahaman SO, Grove LM, Paruchuri S, Southern BD, Abraham S, Niese KA, Scheraga RG, Ghosh S, Thodeti CK, Zhang DX, Moran MM, Schilling WP, Tschumperlin DJ, Olman MA (2014) TRPV4 mediates myofibroblast differentiation and pulmonary fibrosis in mice. J Clin Invest 124(12):5225–5238. https://doi.org/10.1172/JCI75331
Sharma S, Goswami R, Merth M, Cohen J, Lei KY, Zhang DX, Rahaman SO (2017) TRPV4 ion channel is a novel regulator of dermal myofibroblast differentiation. Am J Physiol Cell Physiol 312(5):C562–C572. https://doi.org/10.1152/ajpcell.00187.2016
Goswami R, Merth M, Sharma S, Alharbi MO, Aranda-Espinoza H, Zhu X, Rahaman SO (2017) TRPV4 calcium-permeable channel is a novel regulator of oxidized LDL-induced macrophage foam cell formation. Free Radic Biol Med 110:142–150. https://doi.org/10.1016/j.freeradbiomed.2017.06.004
Adapala RK, Thoppil RJ, Luther DJ, Paruchuri S, Meszaros JG, Chilian WM, Thodeti CK (2013) TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals. J Mol Cell Cardiol 54:45–52. https://doi.org/10.1016/j.yjmcc.2012.10.016
Sharma S, Goswami R, Zhang DX, Rahaman SO (2019) TRPV4 regulates matrix stiffness and TGFβ1-induced epithelial-mesenchymal transition. J Cell Mol Med 23(2):761–774. https://doi.org/10.1111/jcmm.13972
Sharma S, Goswami R, Rahaman SO (2019) The TRPV4-TAZ mechanotransduction signaling axis in matrix stiffness- and TGFβ1-induced epithelial-mesenchymal transition. Cell Mol Bioeng 12:139–152. https://doi.org/10.1007/s12195-018-00565-w
Garcia-Elias A, Mrkonjić S, Jung C, Pardo-Pastor C, Vicente R, Valverde MA (2014) The TRPV4 channel. In: Nilius B, Flockerzi V (eds) Mammalian Transient Receptor Potential (TRP) cation channels. Springer, Berlin, pp 293–319
Everaerts W, Nilius B, Owsianik G (2010) The vanilloid transient receptor potential channel TRPV4: from structure to disease. Prog Biophys Mol Biol 103:2–17. https://doi.org/10.1016/j.pbiomolbio.2009.10.002
Liedtke W, Tobin DM, Bargmann CI, Friedman JM (2003) Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc Natl Acad Sci USA 100(suppl 2):14531–14536. https://doi.org/10.1073/pnas.2235619100
Goswami R, Cohen J, Sharma S, Zhang DX, Lafyatis R, Bhawan J, Rahaman SO (2017) TRPV4 ion channel is associated with scleroderma. J Invest Dermatol 137:962–965. https://doi.org/10.1016/j.jid.2016.10.045
Esteller M (2011) Non-coding RNAs in human disease. Nat Rev Genet 12(12):861–874. https://doi.org/10.1038/nrg3074
Ponting CP, Oliver PL, Reik W (2009) Evolution and functions of long noncoding RNAs. Cell 136:629–641. https://doi.org/10.1016/j.cell.2009.02.006
Hrdlickova B, de Almeida RC, Borek Z (1842) Withoff S (2014) Genetic variation in the non-coding genome: Involvement of micro-RNAs and long non-coding RNAs in disease. Biochim Biophys Acta 10:1910–1922. https://doi.org/10.1016/j.bbadis.2014.03.011
Tao H, Yang JJ, Hu W, Shi KH, Deng ZY, Li J (2016) Noncoding RNA as regulators of cardiac fibrosis: current insight and the road ahead. Pflugers Arch 468(6):1103–1111. https://doi.org/10.1007/s00424-016-1792-y
Miao CG, Xiong YY, Yu H, Zhang XL, Qin MS, Song TW, Du CL (2015) Critical roles of microRNAs in the pathogenesis of systemic sclerosis: new advances, challenges and potential directions. Int Immunopharmacol 28(1):626–633. https://doi.org/10.1016/j.intimp.2015.07.042
Yang LL, Liu JQ, Bai XZ, Fan L, Han F, Jia WB, Su LL, Shi JH, Tang CW, Hu DH (2014) Acute downregulation of miR-155 at wound sites leads to a reduced fibrosis through attenuating inflammatory response. Biochem Biophys Res Commun 453(1):153–159. https://doi.org/10.1016/j.bbrc.2014.09.077
Pandit KV, Milosevic J, Kaminski N (2011) MicroRNAs in idiopathic pulmonary fibrosis. Transl Res 157(4):191–199. https://doi.org/10.1016/j.trsl.2011.01.012
Nicoloso MS, Spizzo R, Shimizu M, Rossi S, Calin GA (2009) MicroRNAs- the micro steering wheel of tumour metastases. Nat Rev Cancer 9:293–302. https://doi.org/10.1038/nrc2619
Svoronos AA, Engelman DM, Slack FJ (2016) OncomiR or tumor suppressor? The duplicity of microRNAs in cancer. Cancer Res 76(13):3666–3670. https://doi.org/10.1158/0008-5472.CAN-16-0359
Schmitz SU, Grote P, Herrmann BG (2016) Mechanisms of long noncoding RNA function in development and disease. Cell Mol Life Sci 73(13):2491–2509. https://doi.org/10.1007/s00018-016-2174-5
Liz J (1859) Esteller M (2016) lncRNAs and microRNAs with a role in cancer development. Biochim Biophys Acta 1:169–176. https://doi.org/10.1016/j.bbagrm.2015.06.015
Suzuki M, Mizuno A, Kodaira K, Imai M (2003) Impaired pressure sensation in mice lacking TRPV4. J Biol Chem 278(25):22664–22668. https://doi.org/10.1074/jbc.M302561200
Zhang DX, Mendoza SA, Bubolz AH, Mizuno A, Ge ZD, Li R, Warltier DC, Suzuki M, Gutterman DD (2009) Transient receptor potential vanilloid type 4-deficient mice exhibit impaired endothelium-dependent relaxation induced by acetylcholine in vitro and in vivo. Hypertension 53(3):532–538. https://doi.org/10.1161/HYPERTENSIONAHA.108.127100
Lichti U, Anders J, Yuspa SH (2008) Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immune-deficient mice. Nat Protoc 3:799–810. https://doi.org/10.1038/nprot.2008.50
Derynck R, Muthusamy BP, Saeteurn KY (2014) Signaling pathway cooperation in TGF-β-induced epithelial-mesenchymal transition. Curr Opin Cell Biol 31:56–66. https://doi.org/10.1016/j.ceb.2014.09.001
O'Connor JW, Riley PN, Nalluri SM, Ashar PK, Gomez EW (2015) Matrix rigidity mediates TGFβ1-induced epithelial-myofibroblast transition by controlling cytoskeletal organization and MRTF-A localization. J Cell Physiol 230(8):1829–1839. https://doi.org/10.1002/jcp.24895
Fatica A, Bozzoni I (2014) Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet 15:7–21. https://doi.org/10.1038/nrg3606
Wang S, Tran EJ (2013) Unexpected functions of lncRNAs in gene regulation. Commun Integr Biol 6(6):e27610. https://doi.org/10.4161/cib.27610
Beermann J, Piccoli MT, Viereck J, Thum T (2016) Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol Rev 96(4):1297–1325. https://doi.org/10.1152/physrev.00041.2015
Richards EJ, Zhang G, Li ZP, Permuth-Wey J, Challa S, Li Y, Kong W, Dan S, Bui MM, Coppola D, Mao WM, Sellers TA, Cheng JQ (2015) Long non-coding RNAs (LncRNA) regulated by transforming growth factor (TGF) β: LncRNA-hit-mediated TGFβ-induced epithelial to mesenchymal transition in mammary epithelia. J Biol Chem 290(11):6857–6867. https://doi.org/10.1074/jbc.M114.610915
Cao G, Zhang J, Wang M, Song X, Liu W, Mao C, Lv C (2013) Differential expression of long non-coding RNAs in bleomycin-induced lung fibrosis. Int J Mol Med 32(2):355–364. https://doi.org/10.3892/ijmm.2013.1404
Li J, Long W, Li Q, Zhou Q, Wang Y, Wang H, Zhou B, Li J (2015) Distinct expression profiles of lncRNAs between regressive and mature scars. Cell Physiol Biochem 35(2):663–675. https://doi.org/10.1159/000369727
Yang S, Yao H, Li M, Li H, Wang F (2016) Long non-coding RNA MALAT1 mediates transforming growth factor beta1-induced epithelial-mesenchymal transition of retinal pigment epithelial cells. PLoS ONE 11(3):e0152687. https://doi.org/10.1371/journal.pone.0152687
Zhou CF, Zhou DC, Zhang JX, Wang F, Cha WS, Wu CH, Zhu QX (2014) Bleomycin-induced epithelial mesenchymal transition in sclerotic skin of mice: possible role of oxidative stress in the pathogenesis. Toxicol Appl Pharmacol 277:250–258. https://doi.org/10.1016/j.taap.2014.03.024
Nikitorowicz-Buniak J, Denton CP, Abraham D, Stratton R (2015) Partially evoked epithelial-mesenchymal transition (EMT) is associated with increased TGFβ signaling within lesional scleroderma skin. PLoS ONE 10:e0134092. https://doi.org/10.1371/journal.pone.0134092
Discher DE, Janmey P, Wang YL (2005) Tissue cells feel and respond to the stiffness of their substrate. Science 310:1139–1143. https://doi.org/10.1126/science.1116995
Duval K, Grover H, Han LH, Mou Y, Pegoraro AF, Fredberg J, Chen Z (2017) Modeling physiological events in 2D vs. 3D cell culture. Physiology (Bethesda) 32:266–277. https://doi.org/10.1152/physiol.00036.2016
Funding
This work was supported by National Institute of Health (Grant No 1R01EB024556-01) and National Science Foundation (CMMI-1662776) Grants to Shaik O. Rahaman.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by SOR, SS, and LM.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, S., Ma, L. & Rahaman, S.O. Role of TRPV4 in matrix stiffness-induced expression of EMT-specific LncRNA. Mol Cell Biochem 474, 189–197 (2020). https://doi.org/10.1007/s11010-020-03844-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03844-1