Abstract
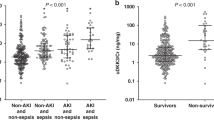
The Wnt signaling pathway has critical roles in dysregulated inflammation during sepsis; however, its impacts on clinical outcomes remain uncertain. This prospective observational study investigated the association between the Wnt pathway and clinical outcomes in patients with urosepsis. The study included 38 patients with urosepsis and 20 healthy individuals. Wnt3a and Wnt5a levels were measured at admission. The primary outcome was the occurrence of major adverse kidney events (MAKE), defined as new renal replacement therapy, stage 3 acute kidney injury, or death. Both Wnt3a and Wnt5a levels were higher in the patient group than in the control (P = 0.001 and P < 0.001, respectively). The primary outcome occurred in 13 (34.2%) subjects. The levels of Wnt5a were higher in subjects with MAKE than in those without MAKE (P = 0.015); however, Wnt3a levels showed no significant difference. Moreover, Wnt5a levels could be a marker to predict the possibility of MAKE (area under the curve 0.74 [0.57–0.92]; P = 0.016). Serum creatinine levels on day 0, day 5, and on discharge day were evaluated. The levels of creatinine on discharge day were higher in patients with high Wnt5a levels, compared to those with low Wnt5a levels (P = 0.030); however, no difference in Wnt5a levels was observed on day 0 and 5. Wnt3a and Wnt5a levels increased in patients with urosepsis. Moreover, evaluation of Wnt5a levels might help to predict the occurrence of MAKE and renal recovery in these patients.






Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, Rubenfeld G, Kahn JM, Shankar-Hari M, Singer M, Deutschman CS, Escobar GJ, Angus DC (2016) Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315(8):762–774. https://doi.org/10.1001/jama.2016.0288
Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C, Beginning, and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators (2005) Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294(7):813–818. https://doi.org/10.1001/jama.294.7.813
Bagshaw SM, Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Oudemans-van Straaten HM, Ronco C, Kellum JA, Beginning, and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators (2007) Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol 2(3):431–439. https://doi.org/10.2215/CJN.03681106
Marshall JC (2014) Why have clinical trials in sepsis failed? Trends Mol Med 20(4):195–203. https://doi.org/10.1016/j.molmed.2014.01.007
Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL (2016) Sepsis and septic shock. Nat Rev Dis Primers 2:16045. https://doi.org/10.1038/nrdp.2016.45
Mogensen TH (2009) Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 22(2):240–273. https://doi.org/10.1128/CMR.00046-08
Cohen J (2002) The immunopathogenesis of sepsis. Nature 420(6917):885–891. https://doi.org/10.1038/nature01326
Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Engl J Med 348(2):138–150. https://doi.org/10.1056/NEJMra021333
Kalakeche R, Hato T, Rhodes G, Dunn KW, El-Achkar TM, Plotkin Z, Sandoval RM, Dagher PC (2011) Endotoxin uptake by S1 proximal tubular segment causes oxidative stress in the downstream S2 segment. J Am Soc Nephrol 22(8):1505–1516. https://doi.org/10.1681/ASN.2011020203
Dellepiane S, Marengo M, Cantaluppi V (2016) Detrimental cross-talk between sepsis and acute kidney injury: new pathogenic mechanisms, early biomarkers and targeted therapies. Crit Care 20:61. https://doi.org/10.1186/s13054-016-1219-3
van Amerongen R, Nusse R (2009) Towards an integrated view of Wnt signaling in development. Development 136(19):3205–3214. https://doi.org/10.1242/dev.033910
Staal FJ, Luis TC, Tiemessen MM (2008) WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol 8(8):581–593. https://doi.org/10.1038/nri2360
Zimmerman ZF, Moon RT, Chien AJ (2012) Targeting Wnt pathways in disease. Cold Spring Harb Perspect Biol 4(11):e008086. https://doi.org/10.1101/cshperspect.a008086
George SJ (2008) Wnt pathway: a new role in regulation of inflammation. Arterioscler Thromb Vasc Biol 28(3):400–402. https://doi.org/10.1161/ATVBAHA.107.160952
Pereira CP, Bachli EB, Schoedon G (2009) The wnt pathway: a macrophage effector molecule that triggers inflammation. Curr Atheroscler Rep 11(3):236–242
Jang J, Jung Y, Kim Y, Jho EH, Yoon Y (2017) LPS-induced inflammatory response is suppressed by Wnt inhibitors, Dickkopf-1 and LGK974. Sci Rep 7:41612. https://doi.org/10.1038/srep41612
Sharma A, Yang WL, Ochani M, Wang P (2017) Mitigation of sepsis-induced inflammatory responses and organ injury through targeting Wnt/beta-catenin signaling. Sci Rep 7(1):9235. https://doi.org/10.1038/s41598-017-08711-6
Pereira C, Schaer DJ, Bachli EB, Kurrer MO, Schoedon G (2008) Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler Thromb Vasc Biol 28(3):504–510. https://doi.org/10.1161/ATVBAHA.107.157438
Schulte DM, Kragelund D, Muller N, Hagen I, Elke G, Titz A, Schadler D, Schumacher J, Weiler N, Bewig B, Schreiber S, Laudes M (2015) The wingless-related integration site-5a/secreted frizzled-related protein-5 system is dysregulated in human sepsis. Clin Exp Immunol 180(1):90–97. https://doi.org/10.1111/cei.12484
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup (2013) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock (2012). Intensive Care Med 39(2):165–228. https://doi.org/10.1007/s00134-012-2769-8
Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A et al (1991) The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest 100(6):1619–1636. https://doi.org/10.1378/chest.100.6.1619
Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S (1998) Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 26(11):1793–1800. https://doi.org/10.1097/00003246-199811000-00016
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group (2012) KDIGO clinical practice guideline for acute kidney injury. Kidney Int 2(suppl):138
Pashirzad M, Shafiee M, Rahmani F, Behnam-Rassouli R, Hoseinkhani F, Ryzhikov M, Moradi Binabaj M, Parizadeh MR, Avan A, Hassanian SM (2017) Role of Wnt5a in the pathogenesis of inflammatory diseases. J Cell Physiol 232(7):1611–1616. https://doi.org/10.1002/jcp.25687
Mehta RL, Bouchard J, Soroko SB, Ikizler TA, Paganini EP, Chertow GM, Himmelfarb J, Program to Improve Care in Acute Renal Disease (PICARD) Study Group (2011) Sepsis as a cause and consequence of acute kidney injury: program to improve care in acute renal disease. Intensive Care Med 37(2):241–248. https://doi.org/10.1007/s00134-010-2089-9
Gruber J, Yee Z, Tolwinski NS (2016) Developmental drift and the role of Wnt signaling in aging. Cancers (Basel) 8(8):73. https://doi.org/10.3390/cancers8080073
Palomer E, Buechler J, Salinas PC (2019) Wnt signaling deregulation in the aging and Alzheimer’s brain. Front Cell Neurosci 13:227. https://doi.org/10.3389/fncel.2019.00227
Xiao L, Zhou D, Tan RJ, Fu H, Zhou L, Hou FF, Liu Y (2016) Sustained activation of Wnt/beta-catenin signaling drives AKI to CKD progression. J Am Soc Nephrol 27(6):1727–1740. https://doi.org/10.1681/ASN.2015040449
Zhou D, Tan RJ, Fu H, Liu Y (2016) Wnt/beta-catenin signaling in kidney injury and repair: a double-edged sword. Lab Invest 96(2):156–167. https://doi.org/10.1038/labinvest.2015.153
Rydell-Tormanen K, Zhou XH, Hallgren O, Einarsson J, Eriksson L, Andersson-Sjoland A, Westergren-Thorsson G (2016) Aberrant nonfibrotic parenchyma in idiopathic pulmonary fibrosis is correlated with decreased beta-catenin inhibition and increased Wnt5a/b interaction. Physiol Rep 4(5):12727
Rashid ST, Humphries JD, Byron A, Dhar A, Askari JA, Selley JN, Knight D, Goldin RD, Thursz M, Humphries MJ (2012) Proteomic analysis of extracellular matrix from the hepatic stellate cell line LX-2 identifies CYR61 and Wnt-5a as novel constituents of fibrotic liver. J Proteome Res 11(8):4052–4064. https://doi.org/10.1021/pr3000927
Abraityte A, Vinge LE, Askevold ET, Lekva T, Michelsen AE, Ranheim T, Alfsnes K, Fiane A, Aakhus S, Lunde IG, Dahl CP, Aukrust P, Christensen G, Gullestad L, Yndestad A, Ueland T (2017) Wnt5a is elevated in heart failure and affects cardiac fibroblast function. J Mol Med (Berl) 95(7):767–777. https://doi.org/10.1007/s00109-017-1529-1
Lameire N, Kellum JA, KDIGO AKI Guideline Work Group (2013) Contrast-induced acute kidney injury and renal support for acute kidney injury: a KDIGO summary (Part 2). Crit Care 17(1):205. https://doi.org/10.1186/cc11455
Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ et al (2007) Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317(5839):803–806. https://doi.org/10.1126/science.1143578
Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA (2007) Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317(5839):807–810. https://doi.org/10.1126/science.1144090
Acknowledgements
This work was supported by the Korean Society of Dialysis Therapy. The authors would like to extend their gratitude to the Korean Society of Dialysis Therapy.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
DJO is a corresponding author. DJO involved in study conception and design; DJO did data collection; JS and YY performed data analysis; JS and DJO contributed to writing original draft preparation; and YY and DJO did supervision.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethics approval
This study was approved by the Institutional Review Board (IRB) of Myongji Hospital (IRB number: MJH 2017-09-018-029).
Consent to participate
Written informed consent was obtained from each subject or his or her legal representative before inclusion into the study.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shin, J., Yoon, Y. & Oh, DJ. Evaluation of the Wnt signaling pathway as a prognostic marker in patients with urosepsis. Mol Cell Biochem 473, 15–23 (2020). https://doi.org/10.1007/s11010-020-03804-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03804-9