Abstract
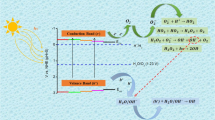
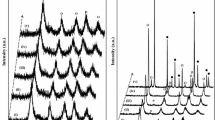
A comparative thermal analysis study was realized by the thermogravimetric and thermodifferential analysis (TG/DTG/DTA) and differential scanning calorimetry (DSC) on TiO2 system. This study is to establish the influence of the preparation method on thermal behavior of powders obtained by different methods as follows: the sol–gel (SG), microwave-assisted sol–gel (MW) and hydrothermal (HT) methods. Titanium (IV) ethoxide, titanium (IV) isopropoxide and titanium (IV) butoxide were used as TiO2 precursors, as well as the parental alcohol as solvent, and the hydrolyzing agent (ammonia containing water) was added drop by drop to obtain a solution with pH 10. Based on TG/DTG/DTA results, the temperature of 450 °C was chosen for the processing of powders in air. Morphology and structure of the calcinated powders were analyzed by scanning electron microscopy (SEM) and X-ray diffraction (XRD). The SEM images show spherical nano-sized particles and X-ray diffractograms for all samples calcinated at 450 °C show crystallization in a single phase of TiO2 (anatase). According to the activation energy, Ea, values calculated by Kissinger and Ozawa methods, amorphous-to-anatase transition is facilitated in the microwaved-assisted samples in comparison with the sol–gel derived samples. The photocatalytic activity of the TiO2 samples toward the degradation of methyl orange (MO) was evaluated, the sample denoted Ti-Bu-SG being the most effective removing a total of ~99% of the dye in 180 min, while Ti-Bu-MW presented the lowest degradation efficiency, about 82%.













Similar content being viewed by others
References
Patil KC, Hegde MS, Rattan T, Aruna ST. Chemistry of nanocrystalline oxide materials. Combustion synthesis, properties and applications. Singapore: World Scientific Publishing Co. Pte. Ltd.; 2008. p. 1–345.
Athar T. Cap. 14, Metal oxide nanopowder and Cap. 17, Smart precursors for smart nanoparticles. In: Ahmed W, Jackson MJ, editors. Emerging nanotechnologies for manufacturing. Norwich: William Andrew Publishing; 2015.
Girish Kumar S, Koteswara Rao KSR. Polymorphic phase transition among the titania crystal structures in solution based approach: from precursor chemistry to nucleation process. Nanoscale. 2014;6:11574–632.
Andrade-Guel M, Díaz-Jiménez L, Cortés-Hernández D, Cabello-Alvaradoc C, Ávila-Ortac C, Bartolo-Pérez P, Gamero-Melo P. Microwave assisted sol–gel synthesis of titanium dioxide using hydrochloric and acetic acid as catalysts. Boletín de la Sociedad Española de Cerámica y Vidrio. 2019;58(4):171–7.
Carp O, Huisman CL, Reller A. Photoinduced reactivity of titanium dioxide. Prog Solid St Chem. 2004;32:33–177.
Byrappa K, Yoshimura M. Handbook of hydrothermal technology. 2nd ed. Norwich: William Andrew Publishing; 2012.
Vinogradov AV, Vinogradov VV. Low-temperature sol–gel synthesis of crystalline materials. J Name. 2013;00:1–3.
Lu CW, Cao Y, Li H, Webb C, Pan WP. Synthesis of TiO2 based on hydrothermal methods using elevated pressures and microwave conditions. J Therm Anal Calorim. 2014;116:1241–8.
Bregadiollia BA, Fernandesa SL, de Graef CFO. Easy and fast preparation of TiO2 - based nanostructures using microwave assisted hydrothermal synthesis. Mater Res. 2017;20(4):912–9.
Falk GS, Borlaf M, López-Muñoz MJ, Farinas JC, Rodrigues Neto JB, Moreno R. Microwave-assisted synthesis of TiO2 nanoparticles: photocatalytic activity of powders and thin films. J Nanopart Res. 2018;20:23.
Morten ES, Li Z, Søgaard EG. Influence of the OH groups on the photocatalytic activity and photoinduced hydrophilicity of microwave assisted sol–gel TiO2 film. Appl Surface Sci. 2009;255(18):8054–62.
Kéri O, Kocsis E, Karajz DA, Nagy ZK, Parditka B, Erdélyi Z, Szabó A, Hernádi K, Szilágyi IM. Photocatalytic crystalline and amorphous TiO2 nanotubes prepared by electrospinning and atomic layer deposition. Molecules. 2021;26:5917.
Akpan UG, Hameed BH. The advancements in sol–gel method of doped-TiO2 photocatalysts - a review. Appl Catal A: Gen. 2010;375:1–11.
Horvat P, Sever Škapin A, Lavrenčič Štangar U, Cerc KR. Thermal techniques as a tool for the direction of the preparation of photocatalytically efficient titania thin films and powders. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-10099-x.
Catauro M, Dal Poggetto G, Risoluti R, Vecchio CS. Thermal, chemical and antimicrobial characterization of bioactive titania synthesized by sol–gel method. J Therm Anal Calorim. 2020;142:1767–74.
Lin W-C, Yang W-D, Jheng S-Y. Photocatalytic degradation of dyes in water using porous nanocrystalline titanium dioxide. J Taiwan Instit Chem Eng. 2012;43:269–74.
Kurajica S, Minga I, Mandić V. Nanocrystalline anatase derived from modified alkoxide mesostructured gel. J Therm Anal Calorim. 2016;124:645–55.
Li X, Wang L, Lu X. Preparation of silver-modified TiO2 via microwave-assisted method and its photocatalytic activity for toluene degradation. J Hazard Mater. 2010;177:639–47.
Tongon W, Chawengkijwanich C, Chiarakorn S. Visible light responsive Ag/TiO2/MCM-41 nanocomposite films synthesized by a microwave assisted sol–gel technique. Superlattices Microstruct. 2014;69:108–21.
Baghbanzadeh M, Carbone L, Cozzoli PD, Kappe CO. Microwave-assisted synthesis of colloidal inorganic nanocrystals. Angew Chem Int Edit. 2011;50:11312–59.
Das S, Mukhopadhyay AK, Datta S, Basu D. Prospects of microwave processing: an overview. B Mater Sci. 2009;32:1–13.
Li Y, Yan W. Microwave synthesis of zeolite membranes: a review. J Membr Sci. 2008;316:3–17.
Canas-Carrell JE, Li S, Parra AM, Shrestha B. Metal oxide nanomaterials: health and environmental effects. In: Njuguna J, Pielichowski K, Zhu H, editors. Health and environmental safety of nanomaterials. Sawston: Woodhead Publishing Limited; 2014. p. 200–21.
Padovan RN, Chubraider X, Azevedo EB, Carvalho LS, Santos Neto ÁJ, Lanças FM, Leitão A, Souza Bergo PL. Degradation of hormones in tap water by heterogeneous solar TiO2-photocatalysis: optimization, degradation products identification, and estrogenic activity removal. J Env Chem Eng. 2021;9:106442. https://doi.org/10.1016/j.jece.2021.106442.
Allen NS, Mahdjoub N, Vishnyakov V, Kelly PJ. KriekRJ, The effect of crystalline phase (anatase, brookite and rutile) and size on the photocatalytic activity of calcined polymorphic titanium dioxide (TiO2). Polym Degrad Stabil. 2018;150:31–6.
Crisan M, Braileanu A, Raileanu M, Crisan D, Teodorescu VS, Birjega R, Marinescu VE, Madarasz J, Pokol G. TiO2-based nanopowders obtained from different Ti-alkoxides. J Therm Anal Calorim. 2007;88:171–6.
Zaharescu M, Crişan M, Crişan D, Gartner M, Moise F. TiO2 films obtained from different Ti–alkoxides. Rev Roum Chim. 1995;40:993–1001.
Zaharescu M, Crişan M, Simionescu L, Crişan D, Gartner M. TiO2 based porous materials obtained from gels, in different experimental conditions. J Sol-Gel SciTechn. 1997;8:249–53.
Crisan M, Braileanu A, Raileanu M, Zaharescu M, Crisan D, Dragan N, Anastasescu M, Ianculescu A, Nitoi I, Marinescu VE, Hodorogea SM. Sol-gel S-doped TiO2 materials for environmental protection. J Non-Cryst Solids. 2008;354:705–11.
Stanciu I, Predoana L, Pandele Cusu J, Preda S, Anastasescu M, Vojisavljević K, Malič B, Zaharescu M. Thermal behaviour of the TiO2 based gels obtained by microwaves assisted sol-gel method. J Therm Anal Calorim. 2017;130(2):639–51.
Stanciu I, Predoana L, Anastasescu C, Culita DC, Preda S, Pandele-Cusu J, Munteanu C, Rusu A, Balint I, Zaharescu M. Structure and properties of Vanadium doped TiO2 powders prepared by sol-gel method. Rev Roum Chim. 2014;59(11–12):921–31.
Li L, Sun X, Yang Y, Guan N, Zhang F. Synthesis of anatase TiO2 nanoparticles with b-cyclodextrin as a supramolecular shell. Chem Asian J. 2006;1:664–6.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29(11):1702–6.
Koga N. Ozawa’s kinetic method for analyzing thermoanalytical curves. J Therm Anal Calorim. 2013;113(3):1527–41.
Zhang Y, Fang ZZ, Sun P, Huang Z, Zheng S. A study on the synthesis of coarse TiO2 powder with controlled particle sizes and morphology via hydrolysis. Powder Technol. 2021;393:650–8.
Wright JD, Sommerdijk NAJM. Sol – gel materials: chemistry and applications. London: CRC Press; 2000.
Poluboyarinov AS, Chelpanov VI, Lebedev VA, Kozlov DA, Khazova KM, Volkov DS, Kolesnik IV, Garshev AV. Titanium oxide microspheres with tunable size and phase composition. Materials. 2019;12(9):1472.
Yeh S-W, Chen Y-L, Hsi C-S, Ko H-H, Wang M-C. Thermal Behavior and Phase Transformation of Tio2 nanocrystallites prepared by a coprecipitation route. Metall Mater Trans. 2014;45A:261–8.
Wang C-L, Hwang W-S, Chu H-L, Lin H-J, Ko H-H, Wang M-C. Kinetics of anatase transition to rutile TiO2 from titanium dioxide precursor powders synthesized by a sol-gel process. Ceram Int. 2016;42:13136–43.
Stojanovi BD, Marinkovi ZV, Brankovi GO, Fidanevska E. Evaluation of kinetic data for crystallization of TiO2 prepared by electrolysis method. J Therm Anal Calorim. 2000;60:595–604.
Vyazovkin S, Burnham AK, Favergeon L, Koga N, Moukhina E, Pérez-Maqueda LA, Sbirrazzuoli N. ICTAC kinetics committee recommendations for analysis of multi-step kinetics. Thermochim Acta. 2020;689: 178597.
Dikici T, Demirci S, Tünçay MM, Yildirim BK, Kaya N. Effect of heating rate on structure, morphology and photocatalytic properties of TiO2 particles: thermal kinetic and thermodynamic studies. J Sol-Gel SciTechn. 2021;97:622–37.
Tripathi A, Singh M, Mathpal MC, Mishra SK, Agarwal A. Study of structural transformation in TiO2 nanoparticles and its optical properties. J Alloy Compd. 2013;549:114–20.
Yu H, Yu J, Cheng B, Lin J. Synthesis, characterization and photocatalytic activity of mesoporous titania nanorod/titanate nanotube composites. J Hazard Mater. 2007;147:581.
Sakai N, Fujishima A, Watanabe T, Hashimoto K. Enhancement of the photoinduced hydrophilic conversion rate of TiO2 film electrode surfaces by anodic polarization. J Phys Chem B. 2001;105:3023–6.
Akram M, Hussain R, Butt FK, Latif M. Study of the effect of microwave holding time on the physicochemical properties of titanium oxide. Mater Res Exp. 2019;6(8): 085041.
Acknowledgements
This work was supported by the research programme “Materials Science and Advanced Methods for Characterization” of “Ilie Murgulescu” Institute of Physical Chemistry, financed by the Roumanian Academy and by a grant from the Romanian Ministry of Research and Innovation, PCCDI-UEFISCDI, project number PN-III-P1-1.2-PCCDI-2017-0476/51PCCDI/2018, within PNCDI III. The authors thank Dr. Maria Elena Anghel from “Ilie Murgulescu” Institute of Physical Chemistry for discussions.
Funding
The authors declare they have no financial interests.
Author information
Authors and Affiliations
Contributions
JP-C contributed to formal analysis, investigation, and writing—original draft; SP contributed to formal analysis, investigation, and writing—original draft; SP contributed to formal analysis, investigation, and writing—original draft; GP contributed to formal analysis and investigation; MC contributed to investigation and writing—original draft; LP contributed to conceptualization, project administration, methodology, writing—original draft, and visualization.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pandele-Cusu, J., Petrescu, S., Preda, S. et al. Comparative study of the TiO2 nanopowders prepared from different precursors and chemical methods for heterogeneous photocatalysis application. J Therm Anal Calorim 147, 13111–13124 (2022). https://doi.org/10.1007/s10973-022-11544-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11544-9