Abstract
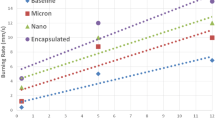
Utilizing metal nitrate salts and sodium hydroxide as a precipitating agent, nanosized nickel-zinc ferrite (NiZnF) particles were successfully synthesized using a co-precipitation route. The effect of a 1% NiZnF additive on the thermal decomposition of AP and the burn rate of AP-based composite solid propellants was investigated. Five isoconversional methods were utilized to determine the activation energy of the AP in the presence of the catalyst using DSC data. An ignition delay study of AP and propellant with and without NiZnF catalyst was carried out using a tube furnace. DSC results suggested that NiZnF exhibits good catalytic activity on the thermal decomposition of AP by decreasing thermal decomposition temperature (90.68 °C) and activation of AP to a great extent.
Graphical abstract











Similar content being viewed by others
References
Sharapa DI, Doronkin DE, Studt F, Grunwaldt JD, Behrens S. Moving frontiers in transition metal catalysis: synthesis. Charact Model Adv Mater. 2019;31:1807381.
Lim CW, Lee IS. Magnetically recyclable nanocatalyst systems for the organic reactions. Nano Today. 2010;5:412–34.
Kharisov BI, Dias HVR, Kharissova OV. Mini-review: ferrite nanoparticles in the catalysis. Arab J Chem. 2019;12:1234–46.
Kaur G, Devi P, Thakur S, Kumar A, Chandel R, Banerjee B. Magnetically separable transition metal ferrites: versatile heterogeneous nano-catalysts for the synthesis of diverse bioactive heterocycles. ChemistrySelect. 2019;4:2181–99.
Cheng T, Zhang D, Li H, Liu G. Magnetically recoverable nanoparticles as efficient catalysts for organic transformations in aqueous medium. Green Chem. 2014;16:3401–27.
Amiri M, Eskandari K, Salavati-Niasari M. Magnetically retrievable ferrite nanoparticles in the catalysis application. Adv Colloid Interface Sci. 2019;271:101982.
Mohallem NDS, Silva JB, Nascimento GLT, Guimarães VL. Study of multifunctional nanocomposites formed by cobalt ferrite dispersed in a silica matrix prepared by sol-gel process. In: Ebrahimi F, editor. Nanocomposites: new trends and developments. London: IntechOpen; 2012. p. 457–81.
Vara JA, Dave PN, Chaturvedi S. Investigating catalytic properties of nanoferrites for both AP and nano-AP based composite solid propellant. Combust Sci Technol. 2021;193:2290–4.
Tsay CY, Chiu YC, Tseng YK. Investigation on structural, magnetic, and FMR properties for hydrothermally-synthesized magnesium-zinc ferrite nanoparticles. Phys B. 2019;570:29–34.
Bakhshi H, Vahdati N, Sedghi A, Mozharivskyj Y. Comparison of the effect of nickel and cobalt cations addition on the structural and magnetic properties of manganese-zinc ferrite nanoparticles. J Magn Magn Mater. 2019;474:56–62.
Yousuf MA, Baig MM, Waseem M, Haider S, Shakir I, Ud-Din Khan S, Warsi MF. Low cost micro-emulsion route synthesis of Cr-substituted MnFe2O4 nanoparticles. Ceram Int. 2019;45:22316–23.
Almessiere MA, Slimani Y, Guner S, Sertkol M, Demir Korkmaz A, Shirsath SE, Baykal A. Sonochemical synthesis and physical properties of Co0.3Ni0.5Mn0.2EuxFe2−xO4 nano-spinel ferrites. Ultrason Sonochem. 2019;58:104654.
Naik MM, Naik HSB, Nagaraju G, Vinuth M, Naika HR, Vinu K. Green synthesis of zinc ferrite nanoparticles in Limonia acidissima juice: characterization and their application as photocatalytic and antibacterial activities. Microchem J. 2019;146:1227–35.
Singh G, Kapoor IPS, Dubey S, Siril PF, Yi JH, Zhao FQ, Hu FQ. Effect of mixed ternary transition metal ferrite nanocrystallites on thermal decomposition of ammmonium perchlorate. Thermochim Acta. 2008;477:42–7.
Singh S, Srivastava P, Singh G. Nanorods, nanospheres, nanocubes: synthesis, characterization and catalytic activity of nanoferrites of Mn, Co, Ni, Part-89. Mater Res Bull. 2013;739–6.
Srivastava P, Kapoor IPS, Singh G. Nanoferrites: preparation, characterization and catalytic activity. J Alloys Compd. 2009;485:88–92.
Boldyrev VV. Thermal decomposition of ammonium perchlorate. Thermochim Acta. 2006;443:1–36.
Rabi B, Ounacer M, Boudad L, Essoumhi A, Sajieddine M, Taibi M, Liba A, Razouk A. Structural, optical and dielectric properties of nickel zinc spinel ferrites synthesized by co-precipitation method. J Mater Sci Mater Electron. 2021;32:932–43.
Singh M, Goyal M, Devlal K. Size and shape effects on the band gap of semiconductor compound nanomaterials. J Taibah Univ Sci. 2018;12:470–5.
Joshi GP, Saxena NS, Mangal R, Mishra A, Sharma TP. Band gap determination of Ni-Zn ferrites. Bull Mater Sci. 2003;26:387–9.
Chakrabarty S, Pal M, Dutta A. Structural, optical and electrical properties of chemically derived nickel substituted zinc ferrite nanocrystals. Mater Chem Phys. 2015;153:221–8.
Nanda KK, Kruis FE, Fissan H, Acet M. Band-gap tuning of PbS nanoparticles by in-flight sintering of size classified aerosols. J Appl Phys. 2002;91:2315–21.
Chai H, Li G, Xiang X, Hu X. Simple preparation of ZnO superstructures self-assembled by hexagonal prisms and their superb catalytic activity in the pyrolysis of ammonium perchlorate. Chem Phys Lett. 2019;730:460–5.
Sing KSW. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl Chem. 1985;57:603–19.
Lazarević ZŽ, Milutinović AN, Jovalekić ČD, Ivanovski VN, Daneu N, Mađarević I, Romčević NŽ. Spectroscopy investigation of nanostructured nickel–zinc ferrite obtained by mechanochemical synthesis. Mater Res Bull. 2015;63:239–47.
Samavati A, Ismail AF. Antibacterial properties of copper-substituted cobalt ferrite nanoparticles synthesized by co-precipitation method. Particuology. 2017;30:158–63.
Kang L, Li S, Wang B, Li X, Zeng Q. Exploration of the energetic material ammonium perchlorate at high pressures: combined Raman spectroscopy and X-ray diffraction study. J Phys Chem C. 2018;122:15937–44.
Zhang M, Zhao F, Yang Y, An T, Qu W, Li H, Zhang J, Li N. Catalytic activity of ferrates (NiFe2O4, ZnFe2O4 and CoFe2O4) on the thermal decomposition of ammonium perchlorate. Propellants Explos Pyrotech. 2020;45:463–71.
Singh G, Kapoor IPS, Dubey S. Bimetallic nanoalloys: preparation, characterization and their catalytic activity. J Alloys Compd. 2009;480:270–4.
Vyazovkin S. Some basics en route to isoconversionalal methodology. In: Vyazovkin S, editor. Isoconversionalal kinetics of thermally stimulated processes. Cham: Springer International Publishing; 2015. p. 1–25.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci Part C Polym Lett. 1966;4:323–8.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Kissinger HE. Variation of peak temperature with heating rate in differential thermal analysis. J Res Natl Bur Stand. 1956;57:217–21.
Akahira T, Sunose T. Method of determining activation deterioration constant of electrical insulating materials. Res Rep Chiba Inst Technol (Sci Technol). 1971;16:22–31.
Starink MJ. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim Acta. 2003;404:163–6.
Trache D, Maggi F, Palmucci I, DeLuca LT. Thermal behavior and decomposition kinetics of composite solid propellants in the presence of amide burning rate suppressants. J Therm Anal Calorim. 2018;132:1601–15.
Quraishi KS, Bustam MA, Krishnan S, Khan MI, Wilfred CD, Lévêque JM. Thermokinetics of alkyl methylpyrrolidinium [NTf2] ionic liquids. J Therm Anal Calorim. 2017;129:261.
Gao Z, Nakada M, Amasaki I. A consideration of errors and accuracy in the isoconversionalal methods. Thermochim Acta. 2001;369:137–42.
Trache D, Abdelaziz A, Siouani B. A simple and linear isoconversionalal method to determine the pre-exponential factors and the mathematical reaction mechanism functions. J Therm Anal Calorim. 2017;128:335–48.
Genieva SD, Vlaev LT, Atanassov AN. Study of the thermooxidative degradation kinetics of poly(tetrafluoroethene) using iso-conversional calculation procedure. J Therm Anal Calorim. 2010;551–1.
Ramdani Y, Liu Q, Huiquan G, Liu P, Wang J. Synthesis, characterization and kinetic computations of fullerene (C60)–CuO on the mechanism decomposition of ammonium perchlorate. Mater Today Chem. 2018;10:19–30.
Singh G, Kapoor IPS, Dubey S, Siril PF. Kinetics of thermal decomposition of ammonium perchlorate with nanocrystals of binary transition metal ferrites. Propellants Explos Pyrotech. 2009;34:78–83.
Chen T, Du P, Jiang W, Liu J, Hao G, Gao H, Xiao L, Ke X, Zhao F, Xuan C. A facile one-pot solvothermal synthesis of CoFe2O4/RGO and its excellent catalytic activity on thermal decomposition of ammonium perchlorate. RSC Adv. 2016;87:83838–47.
Zhao S, Ma D. Preparation of CoFe2O4 nanocrystallites by solvothermal process and its catalytic activity on the thermal decomposition of ammonium perchlorate. J Nanomater. 2010;2010:28–32.
Dey A, Athar J, Varma P, Prasant H, Sikder AK, Chattopadhyay S. Graphene-iron oxide nanocomposite (GINC): an efficient catalyst for ammonium perchlorate (AP) decomposition and burn rate enhancer for AP based composite propellant. RSC Adv. 2015;5:1950.
Han A, Liao J, Ye M, Li Y, Peng X. Preparation of nano-MnFe2O4 and its catalytic performance of thermal decomposition of ammonium perchlorate. Chin J Chem Eng. 2011;19:1047–51.
Wang W, Zhang D. Facile preparation of rGO/MFe2O4 (M = Cu Co, Ni) nanohybrids and its catalytic performance during the thermal decomposition of ammonium perchlorate. RSC Adv. 2018;8:32221.
Song M, Chen M, Zhang Z. Effect of Zn powders on the thermal decomposition of ammonium perchlorate. Propellants Explos Pyrotech. 2008;33:261–5.
Acknowledgements
The authors are grateful to the Department of Chemistry, Sardar Patel University for providing research facility, the Department of Physics, Sardar Patel University for providing Raman facility, and Savitribai Phule Pune University for providing BET, DSC, and TGA facilities. RS and RT are also thankful to DST project no SR/NM/NT-1014/2016 (G) for Junior Research Fellowship and Research Associate fellowship, respectively.
Funding
This study was funded by the Department of Science & Technology (Project No SR/NM/NT-1014/2016 (G)), New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no known conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dave, P.N., Sirach, R. NiZnFe2O4: a potential catalyst for the thermal decomposition of AP and burn rate modifier for AP/HTPB based propellants. J Therm Anal Calorim 147, 10999–11011 (2022). https://doi.org/10.1007/s10973-022-11305-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11305-8