Abstract
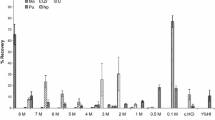
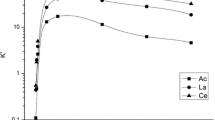
The distribution coefficients of 41 elements were determined on Eichrom’s Actinide resin in HCl and HNO3 solutions of concentrations between 0.01 M and 9 M. A separation scheme for these 41 elements was designed utilizing these distribution coefficients that combines the Actinide resin with Eichrom’s TRU resin. Overall, these values improve the ability to create separations targeting specific analytes utilizing Actinide resin.





Similar content being viewed by others
References
Dry DE, Bauer E, Petersen LA (2005) Rapid separation of fresh fission products. J Radioanal Nucl Chem 263:19–22
Conte E, Widom E, Kuentz D (2017) Uranium isotopes in tree bark as a spatial tracer of environmental contamination near former uranium processing facilities in southwest Ohio. J Environ Radioact 178–179:265–278
Gonzales ER, Garcia SR, Mahan C, Hang W (2005) Evaluation of mass spectrometry and radiation detection for the analysis of radionuclides. J Radioanal Nucl Chem 263:457–465
Hanson DE, Garrison JR, Hall HL (2011) Assessing thermochromatography as a separation method for nuclear forensics: current capability vis-à-vis forensic requirements. J Radioanal Nucl Chem 289:213–223
Stanley FE, Stalcup AM, Spitz HB (2012) A brief introduction to analytical methods in nuclear forensics. J Radioanal Nucl Chem 295:1385–1393
Horwitz EP, Chiarizia R, Dietz ML (1997) DIPEX: a new extraction chromatographic material for the separation and preconcentration of actinides from aqueous solution. React Funct Polym 33:25–36
Horwitz EP, Dietz ML, Chiarizia R, Diamond H, Maxwell SL, Nelson MR (1995) Separation and preconcentration of actinides by extraction chromatography using a supported liquid anion-exchanger—application to the characterization of high-level nuclear waste solutions. Anal Chim Acta 310:63–78
Horwitz EP, Dietz ML, Chiarizia R, Diamond H, Essling AM, Graczyk D (1992) Separation and preconcentration of uranium from acidic media by extraction chromatography. Anal Chim Acta 266:25–37
Horwitz EP, McAlister DR (2005) The separation of beryllium from selected elements using the Dipex®Extraction chromatographic resin. Solvent Extr Ion Exch 23:611–629
Nguyen TH, Lee MS (2015) Separation of molybdenum(VI) and tungsten(VI) from sulfuric acid solution by ion exchange with TEVA resin. Sep Sci Technol 50:2060–2065
Snow MS, Finck MR, Carney KP, Morrison SS (2017) Extraction chromatographic separations of tantalum and tungsten from hafnium and complex matrix constituents. J Chromatogr A 1484:1–6
Barrett KE, Aluicio-Sarduy E, Happel S, Olson AP, Kutyreff CJ, Ellison PA, Barnhart TE, Engle JW (2021) Characterization of actinide resin for separation of 51,52gMn from bulk target material. Nucl Med Biol 96–97:19–26
Makishima A, Zhu X-K, Belshaw NS, O’Nions RK (2002) Separation of titanium from silicates for isotopic ratio determination using multiple collector ICP-MS. J Anal At Spectrom 17:1290–1294
Shimada A, Ozawa M, Yabuki K, Kimiyama K, Sato K, Kameo Y (2014) Development of a separation method for molybdenum from zirconium, niobium, and major elements of rubble samples. J Chromatogr A 1371:163–167
Ekatova TY, Kazakov AG (2019) Extraction-chromatographic behavior of Zr(IV) and Hf(IV) on TRU and LN resins in mixtures of HNO3 and HF. J Radioanal Nucl Chem 321:557–563
Grahek Ž, RožmarićMačefat M (2005) Extraction chromatographic separation of iron from complex liquid samples and the determination of 55Fe. J Radioanal Nucl Chem 267:131–137
Guérin N, Riopel R, Kramer-Tremblay S, de Silva N, Cornett J, Dai X (2017) Extraction of Tc(VII) and Re(VII) on TRU resin. Radiochim Acta 105:197–204
Croudace IW, Warwick PE, Greenwood RC (2006) A novel approach for the rapid decomposition of Actinide™ resin and its application to measurement of uranium and plutonium in natural waters. Anal Chim Acta 577:111–118
Burnett WC, Corbett DR, Schultz M, Horwitz EP, Chiarizia R, Dietz M, Thakkar A, Fern M (1997) Pre-concentration of actinide elements from soils and large volume water samples using extraction chromatography. J Radioanal Nucl Chem 226:121–127
Binnie SA, Dunai TJ, Voronina E, Goral T, Heinze S, Dewald A (2015) Separation of Be and Al for AMS using single-step column chromatography. Nucl Instrum Methods Phys Res Sect B 361:397–401
Beck CL, Herman SM, Warzecha EJ, Emerson HP, Stene RE, Haney MM, Seiner BN, and Metz LA (2022) Separation of select metal isotopes from a mixed activation and fission product sample. J Radioanal Nucl Chem
Varga Z, Katona R, Stefanka Z, Wallenius M, Mayer K, Nicholl A (2010) Determination of rare-earth elements in uranium-bearing materials by inductively coupled plasma mass spectrometry. Talanta 80:1744–1749
Egorov O, Grate JW, Ruzicka J (1998) Automation of radiochemical analysis by flow injection techniques: Am-Pu separation using TRU-resin™ sorbent extraction column. J Radioanal Nucl Chem 234:231–235
Quidelleur S, Granet M, Laszak I, Isnard H, Pons-Branchu E, Brennetot R, Caussignac C (2009) One step U-Pu-Cs-Ln-steel separation using TRU preconditioned extraction resins from Eichrom for application on transmutation targets. J Radioanal Nucl Chem 280:507–517
Grahek Ž, Mačefat MR (2004) Isolation of iron and strontium from liquid samples and determination of 55Fe and 89,90Sr in liquid radioactive waste. Anal Chim Acta 511:339–348
Arrigo LM, Greenwood LR, Pierson BD, Metz LA, Friese JI (2018) Radiochemical separations and experimental measurements of short-lived fission products from 14 MeV irradiation of depleted uranium. J Radioanal Nucl Chem 318:353–360
Morley SM, Seiner B, Finn E, Greenwood L, Smith SC, Gregory S, Haney M, Lucas D, Arrigo L, Beacham T, Swearingen K, Friese J, Douglas M, Metz L (2014) Integrated separation scheme for measuring a suite of fission and activation products from a fresh mixed fission and activation product sample. J Radioanal Nucl Chem 304:509–515
Douglas M, Friese JI, Greenwood LR, Farmer OT, Thomas ML, Maiti TC, Finn EC, Garofoli SJ, Gassman PL, Huff MM, Schulte SM, Smith SC, Thomas KK, and Bachelor PP (2009) Separation and quantification of chemically diverse analytes in neutron irradiated fissile materials. J Radioanal Nuclear Chem 282
Arrigo LM, Jiang J, Finch ZS, Bowen JM, Herman SM, Greenwood LR, Friese JI, Seiner BN (2021) Separation of lanthanide isotopes from mixed fission product samples. Separations 8:104
Metz LA, Friese JI, Finn EC, Greenwood LR, Kephart RF, Hines CC, King MD, Henry KM, Wall DE (2012) Fission yield measurements from highly enriched uranium irradiated inside a boron carbide capsule. J Radioanal Nucl Chem 296:763–767
Chiarizia R, Horwitz EP, Alexandratos SD, Gula MJ (1997) Diphonix(R) resin: a review of its properties and applications. Sep Sci Technol 32:1–35
Ichikawa F, Uruno S, Imai H (1961) Distribution of Various Elements between Nitric Acid and Anion Exchange Resin. Bull Chem Soc Jpn 34:952–955
Strelow FWE (1984) Distribution coefficients and ion exchange behavior of 46 elements with a macroreticular cation exchange resin in hydrochloric acid. Anal Chem 56:1053–1056
Strelow FW (1978) Distribution coefficients and anion exchange behavior of some elements in hydrobromic-nitric acid mixtures. Anal Chem 50:1359–1361
Korkisch J (2017) Handbook of ion exchange resins: their application to inorganic analytical chemistry, vol VI
Meinhard JE (1949) Chromatography: a perspective. Science 110:387–392
Vermeulen T, Hiester NK (1952) Ion-exchange chromatography of trace component—a design theory. Ind Eng Chem 44:636–651
Snow M, Ward J (2020) Fundamental distribution coefficient data and separations using eichrom extraction chromatographic resins. J Chromatogr A 1620:460833
Pourmand A, Dauphas N (2010) Distribution coefficients of 60 elements on TODGA resin: application to Ca, Lu, Hf, U and Th isotope geochemistry. Talanta 81:741–753
Eikenberg J, Jaggi M, Beer H, Ruthi M, Zumsteg I (2009) Separation techniques for low-level determination of actinides in soil samples. Appl Radiat Isot 67:776–780
Acknowledgements
This work was supported by the Office of Defense Nuclear Nonproliferation Research and Development within the U.S. Department of Energy’s National Nuclear Security Administration and Pacific Northwest National Laboratory, which is operated by Battelle Memorial Institute for the U.S. Department of Energy under contract DE-AC05-76RLO1830
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Warzecha, E., Herman, S.M., Arnold, E. et al. Determination of distribution coefficients of 41 elements in nitric and hydrochloric acids for actinide resin. J Radioanal Nucl Chem 332, 2785–2791 (2023). https://doi.org/10.1007/s10967-023-08931-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08931-3