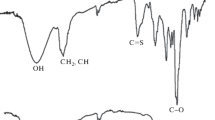
Abstract
A novel reversible addition fragmentation transfer (RAFT) agent based on the poly-3-hydroxy butyrate (PHB) with three hydroxyl groups (PHB-R2) and thermoresponsive amphiphilic block copolymers derived from N-isopropyl acryl amide (NIPAM) are described. Hydroxylated PHB is obtained by the reaction between PHB and diethanol amine (DEA) to prepare hydroxylated PHB (PHB-DEA). It is then reacted with a RAFT agent, 2-(dodecylthiocarbonothioylthio)-2-methylpropionic acid (DDMAT). Hydroxyl ends of the hydroxylated PHB are capped with carboxylic acid end of the trithiocarbonate. The block copolymers obtained by the polymerization of NIPAM initiated by PHB-R2 were characterized by 1H NMR and physicochemical techniques. PHB content in the obtained block copolymers is varying between 14 and 45 wt%. The thermo-responsive PHB-PNIPAM block copolymers show the lower critical solution temperature (LCST) 28 and 30 °C while LCST of the pure PNIPAM is 32 °C.

Novel poly(3-hydroxy butyrate) macro RAFT agent. Synthesis and characterization of thermoresponsive block copolymers.











Similar content being viewed by others
References
Lenz RW, Marchessault RH (2005) Bacterial polyesters: biosynthesis, biodegradable plastics and biotechnology. Biomacromolecules 6:1–8
Sudesh K, Abe H, Doi Y (2000) Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog Polym Sci 25:1503–1555
Kocer H, Borcakli M, Demirel S, Hazer B (2003) Production of bacterial polyesters from some various new substrates by Alcaligenes eutrophus and Pseudomonas oleovorans. Turkish J Chem 27:365–374
Ashby RD, Foglia TA (1998) Poly (hydroxyalkanoate) biosynthesis from triglyceride substrates. Poly (hydroxyalkanoate) biosynthesis from triglyceride substrates. Appl Microbiol Biotechnol 49:431–437
Koray O, Koksal MS, Hazer B (2010) Simple production experiment of poly (3-hydroxy butyrate) for science laboratories and its importance for science process skills of prospective teachers. Energy Educ Sci Technol Part B-Social Educational Studies 2(1–2):39–54
Chen GQ (2009) A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem Soc Rev 38:2434–2446
Gross RA, DeMello C, Lenz RW, Brandl H, Fuller RC (1989) The biosynthesis and characterization of poly(β-hydroxyalkanoates) produced by Pseudomonas oleovorans. Macromolecules 22:1106–1115
Hazer B, Lenz RW, Fuller RC (1994) Biosynthesis of methyl branched poly (β-hydroxy alkanoate)s with Pseudomonas oleovorans. Macromolecules 27:45–49
Li X, Loh XJ, Wang K, He C, Li J (2005) Poly(ester urethane)s consisting of poly[(R)-3-hydroxybutyrate] and poly(ethylene glycol) as candidate biomaterials: characterization and mechanical property study. Biomacromolecules 6:2740–2747
Michalak M, Kurcok P, Hakkarainen M (2017) Polyhydroxyalkanoate-based drug delivery systems. Polym Int 66:617–622
Hazer B, Steinbüchel A (2007) Increased diversification of polyhydroxyalkanoates by modification reactions for industrial and medical applications. Appl Microbiol Biotechnol 74:1–12
Hazer DB, Kilicay E, Hazer B (2012) Poly (3-hydroxyalkanoate)s: diversification and biomedical applications. A state of the art review. Mater Sci Eng C 32:637–647
Hazer DB, Hazer B (2011) The effect of gold clusters on the autoxidation of poly (3-hydroxy 10-undecenoate-co-3-hydroxy octanoate) and tissue response evaluation. J Polym Res 18:251–262
Hazer DB, Bal E, Nurlu G, Benli K, Balci S, Öztürk F, Hazer B (2013) In vivo application of poly-3-hydroxyoctanoate as peripheral nerve graft. J Zhejiang University SCIENCE B 14(11):993–1003
Rai R, Keshavarz T, Roether JA, Boccaccini AR, Roy I (2011) Medium chain length polyhydroxyalkanoates, promising new biomedical materials for the future. Mater Sci Eng R Rep 72:29–47
Raza ZA, Riaz S, Banat IM (2018) Polyhydroxyalkanoates: properties and chemical modification approaches for their functionalization. Biotechnol Prog 34:29–41
Hazer B (2015) Simple synthesis of amphiphilic poly (3-hydroxy alkanoate)s with pendant hydroxyl and carboxylic groups via thiol-ene photo click reactions. Polym Degrad Stab 119:159–166
Le Fer G, Babinot J, Versace D-L, Langlois V, Renard E (2012) The amino end groups of Jeffamine were converted into thiol by a reaction with N-acetyl homocysteine thiolactone and subsequently photo grafted. Macromol Rapid Commun 33:2041–2045
Domenek S, Langlois V, Renard E (2007) Bacterial polyesters grafted with poly (ethylene glycol): behaviour in aqueous media. Polym Degrad Stab 92:1384–1392
Kılıçay E, Hazer B, Çoban B, Scholz C (2010) Synthesis and characterization of the poly (ethylene glycol) grafted unsaturated microbial polyesters. Hacettepe J Biol Chem 38:9–17
Jain-Beuguel C, Xue L, Houel-Renault L, Modjinou T, Simon-Colin C, Gref R, Renard E, Langlois V (2019) Water-soluble poly(3-hydroxyalkanoate) sulfonate: versatile biomaterials used as coatings for highly porous nano-metal organic framework. Biomacromolecules 2019(20):3324–3332
Chen GQ, Albertsson AC (2019) Polyhydroxy alkanoates and other biopolymers. Biomacromolecules 20:3211–3212
Sparks J, Scholz C (2008) Synthesis and characterization of a cationic poly (β-hydroxy alkanoate). Biomacromolecules 9:2091–2096
Ma YM, Wei DX, Yao H, Wu LP, Chen GQ (2016) Synthesis, characterization and application of thermoresponsive polyhydroxyalkanoate-graft-poly(n-isopropylacrylamide). Biomacromolecules 17:2680–2690
Cakmakli B, Hazer B, Borcakli M (2001) Poly (styrene peroxide) and poly (methyl methacrylate peroxide) for grafting on unsaturated bacterial polyesters. Macromol Biosci 1(8):348–354
Arkin AH, Hazer B, Borcakli M (2000) Chlorination of poly-3-hydroxy alkanoates containing unsaturated side chains. Macromolecules 33:3219–3223
Arkin AH, Hazer B (2002) Chemical modification of chlorinated microbial polyesters. Biomacromolecules 3(6):1327–1335
Yalcin B, Cakmak M, Arkın AH, Hazer B, Erman B (2006) Control of optical anisotropy at large deformations in PMMA/chlorinated-PHB (PHB-cl) blends: Mechano-optical behavior. Polymer 47:8183–8193
Hazer B (2010) Amphiphilic poly (3-hydroxy alkanoate)s: potential candidates for medical applications. Int J Polym Sci 2010:423460. https://doi.org/10.1155/2010/423460
Loh XJ, Zhang Z-X, Wu Y-L, Lee TS, Li J (2009) Synthesis of novel biodegradable thermoresponsive triblock copolymers based on poly[(r)-3-hydroxybutyrate] and poly(n-isopropylacrylamide) and their formation of thermoresponsive micelles. Macromolecules 42:194–202
Wang K, Liow SS, Wu Q, Li C, Owh C, Li Z, Loh XJ, Wu Y-L (2017) Codelivery for paclitaxel and bcl-2 conversion gene by phb-pdmaema amphiphilic cationic copolymer for effective drug resistant cancer therapy. Macromol Biosci 17:1700186
Reeve MS, McCarthy SP, Gross RA (1990) Chemical degradation of bacterial polyesters for use in the preparation of new degradable block copolymers. Prepr Am Chem Soc Div Polym Sci 31(1):437–438
Hirt TD, Neuenschwander P, Suter UW (1996) Telechelic diols from poly [(R)-3-hydroxybutyric acid] and poly([(R)-3-hydroxybutyric acid]-co-[(R)-3-hydroxyvaleric acid]. Macromol Chem Phys 197: 1609–1614
Hazer B, Akyol E, Şanal T, Guillaume S, Çakmakli B, Steinbuchel A (2019) Synthesis of novel biodegradable elastomers based on poly [3 – hydroxy butyrate] and poly [3-hydroxy octanoate] via transamidation reaction. Polym Bull 2019(76):919–932
Erol A, Rosberg DBH, Hazer B, Göncü BS (2020) Biodegradable and biocompatible radiopaque iodinated poly-3-hydroxy butyrate. Synthesis, characterization and in vitro/in vivo x-ray visibility. Polym Bull. https://doi.org/10.1007/s00289-019-02747-6
Neugebauer D, Rydz J, Goebel I, Dacko P, Kowalczuk M (2007) Synthesis of graft copolymers containing biodegradable poly (3-hydroxybutyrate). Macromolecules 40:1767–1773
Hazer B, Arslan H, Senemoğlu Y, Şen Ş (2019) Synthesis of block/graft copolymers based on vinyl benzyl chloride via reversible addition fragmentation chain transfer (RAFT) polymerization using the carboxylic acid functionalized trithiocarbonate. J Polym Res 26:101, 1-19
Neises B, Steglich W (1978) Simple method for the esterification of carboxylic acid. Chem Int Ed 17:522–524
Cheng J, Wang J (2009) Syntheses of amphiphilic biodegradable copolymers of poly(ethyl ethylene phosphate) and poly(3-hydroxybutyrate) for drug delivery. Sci China Ser B Chem 52(7):961–968
Convertine AJ, Ayres N, Scales CW, Lowe AB, McCormick CL (2004) Facile, controlled, room-temperature RAFT polymerization of N-isopropylacrylamide. Biomacromolecules 5:1177–1180
Bandelli D, Weber C, Schubert US (2019) Strontium isopropoxide: a highly active catalyst for the ring-opening polymerization of lactide and various lactones. Macromol Rapid Commun 40:1900306
Feil H, Bae YH, Feijen J, Kim SW (1993) Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-Isopropylacrylamide copolymers. Macromolecules 26:2496–2500
Liu Y-Y, Lu J, Shao Y-H (2006) Preparation and characterization of poly(N-isopropylacrylamide)-modified poly(2-hydroxyethyl acrylate) hydrogels by interpenetrating polymer networks for sustained drug release. Macromol Biosci 6:452–458
Curley J, Hazer B, Lenz RW, Fuller RC (1996) Production of poly (3-hydroxyalkanoates) containing aromatic substituents by Pseudomonas oleovorans. Macromolecules 29:1762–1766
Toraman T, Hazer B (2014) Synthesis and characterization of the novel thermoresponsive conjugates based on poly (3-hydroxy alkanoates). J Polym Environ 22:159–166
Acknowledgments
This work was supported by the Kapadokya University Research Funds (#KÜN.2018-BAGP-001) and Bülent Ecevit University Research Funds (#BEU-2017-72118496-01). The Authors thank to S. Hamadi (GPC) for technical assistance.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hazer, B., Eren, M., Senemoğlu, Y. et al. Novel poly(3-hydroxy butyrate) macro RAFT agent. Synthesis and characterization of thermoresponsive block copolymers. J Polym Res 27, 147 (2020). https://doi.org/10.1007/s10965-020-02133-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-020-02133-1