Abstract
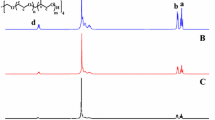
A series of amphiphilic four-arm star polymers poly(e-caprolactone)-b-poly(2-(diethylamino)ethylmethacrylate) (4AS-PCL-b-PDMAEMA) were developed by a combination of ring opening polymerization (ROP) and continuous activators regenerated by electron transfer atom transfer radical polymerization (ARGET ATRP). The molecular structures of the copolymers were confirmed with 1H NMR, FT-IR and gel permeation chromatography (GPC). The critical micelle concentration (CMC) values of the star polymers in aqueous solution were extremely low (3.0–4.1 mg/L).In aqueous solution, the copolymers self-assemble into blank and doxorubicin (DOX)-loaded micelles with an average size of 56–150 nm measured by scanning electron microscopy (SEM) and dynamic light scattering (DLS), which consist of outer shell with blocks PDMAEMA and inner core with blocks PCL. The core block PCL was used to contain drug molecules and shell block PDMAEMA could protonize responding to acidic condition. The in vitro release behavior showed that the micelle structure remains basically unchanged, and the drug cumulative release was only 24.5% at pH 7.4, and at pH 5.0, PDMAEMA is protonated and the micelles swell, which accelerates the release rate of DOX and the cumulative release is higher than pH 7.4. Through the semi-empirical model, the mechanism of drug release process was analyzed, and the mechanism of drug release from polymer micelles was further explored. The in vitro cytotoxicity indicated that the 4AS-PCL-b-PDMAEMA materials had low toxicity, but the DOX-loaded micelles had an obvious inhibitory effect on the HepG2 cells. The results indicate the four-arm star polymers 4AS-PCL-b-PDMAEMA may be a potential drug carriers for pH-triggered DOX release.

self-assembly of the copolymer 4AS-PCL-b-PDMAEMA and the drug loading and pH-responsive release.












Similar content being viewed by others
References
Chen W, Zhou S, Ge L, Wu W, Jiang X (2018) Translatable high drug-loading drug delivery systems based on biocompatible polymer Nanocarriers. Biomacromolecules. 19:1732–1745
Sun X, Guo WW, Hao Z, Shi QH, Xin L, Jian BT, You QS (2018) The blood clearance kinetics and pathway of polymeric micelles in Cancer drug delivery. ACS Nano 12:6179–6192
Samad AA, Bethry A, Janouskova O, Ciccione J, Wenk C, Coll JU, Subra G, Etrych T, Omar FE, Bakkour Y (2017) Iterative Photoinduced chain functionalization as a generic platform for advanced polymeric drug delivery systems. Macromol. Rapid Commun. 39:1700502
Fenton OS, Olafson KN, Pillai PS, Mitchell MJ, Langer R (2018) Advances in biomaterials for drug delivery. Adv. Mater. 30:1705328
Masood F (2016) Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 60:569–578
Zhang M, Song CC, Su S, Du FS, Li ZC (2018) ROS-Activated Ratiometric Fluorescent Polymeric Nanoparticles for Self-Reporting Drug Delivery. ACS Appl. Mater. Interfaces 10:7798–7810
Thambi T, Park JH, Lee DS (2015) Stimuli-responsive polymersomes for cancer therapy. Biomater. Sci. 4:55–69
Deng Y, Ling J, LI MH (2018) Physical stimuli-responsive liposomes and polymersomes as drug delivery vehicles based on phase transitions in the membrane. Nanoscale. 10:6781–6800
Li Y, Bui QN, Le TMD, Yang HY, Lee DS (2018) One-step preparation of pH-responsive polymeric Nanogels as intelligent drug delivery Systems for Tumor Therapy. Biomacromolecules. 19:2062–20700
Poudel AJ, He F, Huang L, Xiao L, Yang G (2018) Supramolecular hydrogels based on poly (ethylene glycol)-poly (lactic acid) block copolymer micelles andα-cyclodextrin for potential injectable drug delivery system. Carbohydr. Polym. 194:69–79
Yi Y, Lin G, Chen S, Liu J, Zhang H, Mi P (2017) Polyester micelles for drug delivery and cancer theranostics: current achievements, progresses and future perspectives. Mater. Sci. Eng. C 83:218–232
Xiong D, Yao N, Gu H, Wang J, Zhang L (2017) Stimuli-responsive shell cross-linked micelles from amphiphilic four-arm star copolymers as potential nanocarriers for “pH/redox-triggered” anticancer drug release. Polymer 114:161–172
Yang YQ, Lin WJ, Zhao B, Wen XF, Guo XD, Zhang LJ (2012) Synthesis and physicochemical characterization of Amphiphilic Triblock copolymer brush containing pH-sensitive linkage for Oral drug delivery. Langmuir the ACS J. Surf. Coll. 28:8251–8259
Lu Y, Yue ZG, Xie JB, Wei W, Zhu H, Zhang ES, Cao ZQ (2018) Micelles with ultralow critical micelle concentration as carriers for drug delivery. Nat. Biomed. Eng. 2:318–325
Katarzyna Jelonek A (2019) Z., Adam Wilczok, Małgorzata Latocha, Monika; Musiał-Kulik, A. F., Janusz Kasperczyk, dual-targeted biodegradable micelles for anticancer drug delivery. Mater. Lett. 241:187–189
Shi XX, Bai S, Yang CJ, Ma XQ, Hou ML, Chen JC, Xue P, Li CM, Kang YJ, Xu ZG (2018) Improving the carrier stability and drug loading of Unimolecular micelle-based Nanotherapeutic for acid-actived drug delivery and enhanced antitumor therapy. J. Mater. Chem. B 6:5549–5561
Giuseppe Tripodo SP, Grisoli P, Trapani A, Maria, Luisa Torre DM (2019) Drug delivery of rifampicin by natural micelles based on inulin: physicochemical properties, antibacterial activity and human macrophages uptake. Eur. J. Pharm. Biopharm. 136:250–258
Chen L, Zang FC, Wu HA, Li JZ, Xie J, Ma M, Gu N, Zhang Y (2018) Using PEGylated magnetic nanoparticles to describe EPR effect in tumor for predicting therapeutic efficacy of micelle drugs. Nanoscale. 10:1788–1797
Hossam S. E-S, Ahmed M. A-A, Tarek A. A, Khalid M. E-S (2018) Stimuli-responsive Nano-architecture drug-delivery systems to solid tumor micromilieu: past, present, and future perspectives. ACS Nano 12, 10636–10664
Chen Q, Zheng J, Yuan X, Wang J, Zhang L (2018) Folic acid grafted and tertiary amino based pH-responsive pentablock polymeric micelles for targeting anticancer drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 82:1–9
Yang L, Sun H, Liu Y, Hou W, Yang Y, Cai R, Cui C, Zhang P, Pan X, Li X (2018) Self-assembled aptamer-hyperbranched polymer nanocarrier for targeted and photoresponsive drug delivery. Angew. Chem. Int. Ed. 130:17294–17298
Zhang L, Qin Y, Zhang Z, Fan F, Huang C, Lu L, Wang H, Jin X, Zhao H, Kong D (2018) Dual pH/reduction-responsive hybrid polymeric micelles for targeted chemo-photothermal combination therapy. Acta Biomater. 75:371–385
Mei L, Rao J, Liu Y, Li M, Zhang Z, He Q (2018) Effective treatment of the primary tumor and lymph node metastasis by polymeric micelles with variable particle sizes. J. Control. Release 292:67–77
Wang Z, Deng XP, Ding JS, Zhou WH, Zheng X, Tang GT (2018) Mechanisms of drug release in pH-sensitive micelles for tumour targeted drug delivery system: a review. Int. J. Pharm. 535:253–260
Zhou Q, Li Z, Tiehong Y, Hong W (2018) Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int. J. Nanomedicine 13:2921–2942
Qirui Tian CF (2019) Hongyao yin, Yujun Feng, stimuli-responsive polymer wormlike micelles. Prog. Polym. Sci. 89:108–132
Can Sarisozen U (2019) J., Livia Palmerston Mendes, Vladimir P. Torchilin, stimuli-responsive polymeric micelles for extracellular and intracellular drug delivery. Stim. Respon. Polym. Nanocarr. Drug Del. Appl. 2:269–304
Yang ZL, Fu KY, Yu J, Zhou PT, Cheng ZH (2018) “pH-triggered” drug release using shell cross-linked micelles from aqueous RAFT-synthesized PAPMA-b-PNIPAM copolymers. J. Polym. Res. 25:164
Sun W, Wen Y, Thiramanas R, Chen MJ, Han JX, Gong NQ, Wagner M, Jiang S, Meijer MS, Bonnet S, Butt HJ, Mailänder V, Liang XJ, Wu S (2018) Red-Light-Controlled Release of Drug-Ru Complex Conjugates from Metallopolymer Micelles for Phototherapy in Hypoxic Tumor Environments. Adv. Funct. Mater. 28:1804227
Zhang YJ, Guo ZY, Cao ZL, Zhou WX, Zhang Y, Chen QJ, Lu YF, Chen XL, Guo Q, Li C, Liang DH, Sun T, Jiang C (2018) Endogenous albumin-mediated delivery of redox-responsive paclitaxel-loaded micelles for targeted cancer therapy. Biomaterials. 183:243–257
Albayaty YN, Thomas N, Jambhrunkar M, AlHawwas M, Anita Kral CRT, Prestidge CA (2019) Enzyme responsive copolymer micelles enhance the anti-biofilm efficacy of the antiseptic chlorhexidine. Int. J. Pharm. 566:329–341
Cao Z, Hao W, Jie D, Wang G (2014) Quadruple-stimuli-sensitive polymeric Nanocarriersfor controlled release under combined stimulation. Macromolecules 47(24):8777–8783
Nayeleh Deirram CZ, Kermaniyan SS, Johnston APR, Such GK (2019) pH-Responsive Polymer Nanoparticles for Drug Delivery. Macromol. Rapid Commun. 40:1800917
Xin DG, Zhang LJ, Chen Y, Qian Y (2010) Core/shell pH-sensitive micelles self-assembled from cholesterol conjugated oligopeptides for anticancer drug delivery. AICHE J. 56:1922–1931
Murphy RF, Powers S, Cantor CR (1984) pH measured in single cells by dual fluorescence flow cytometry: rapid acidification of insulin to pH 6. J. Cell Biol. 98:1757–1762
Gerweck LE, Seetharaman K (1996) Cellular pH gradient in tumor versus Normal tissue: potential exploitation for the treatment of Cancer. Cancer Res. 56:1194–1198
Yamagata M, Hasuda K, Stamato T, Tannock I The contribution of lactic acid to acidification of tumours: studies of variant cells lacking lactate dehydrogenase. Br. J. Cancer 77:1726–1731
Min KH, Kim J-H, Bae SM, Shin H, Kim MS, Park S, Lee H, Park R-W, Kim I-S, Kim K Tumoral acidic pH-responsive MPEG-poly(β-amino ester) polymeric micelles for cancer targeting therapy. J. Control. Release 144:259–266
Wang Z, Li X, Wang D, Zou Y, Qu X, He C, Deng Y, Jin Y, Zhou Y, Zhou Y (2017) Concurrently suppressing multidrug resistance and metastasis of breast cancer by co-delivery of paclitaxel and honokiol with pH-sensitive polymeric micelles. Acta Biomater. 62:144–156
Peeler DJ, Thai SN, Cheng Y, Horner PJ, Sellers DL, Pun SH (2018) pH-sensitive polymer micelles provide selective and potentiated lytic capacity to venom peptides for effective intracellular delivery. Biomaterials 192:235–244
Buwalda S, Al Samad A, El Jundi A, Bethry A, Bakkour Y, Coudane J, Nottelet B (2017) Stabilization of poly(ethylene glycol)-poly(ε-caprolactone) star block copolymer micelles via aromatic groups for improved drug delivery properties. J. Colloid Interface Sci. 514:468–478
Urjita S, Anita B (2018) In-vitro evaluation of cytotoxic and antioxidant properties of drugs solubilized in EO-PO star block copolymer micelles. Colloids Surf. B: Biointerfaces 171:343–350
Shan Zhang YH, Chen H, Liao Z, Chen J, Xu BB, Kong J (2018) Reduction-responsive amphiphilic star copolymers with long-chain hyperbranched poly(ε-caprolactone) core and disulfide bonds for trigger release of anticancer drugs. Eur. Polym. J. 108:364–372
Siepmann J, Peppas NA (2012) Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 64:163–174
Ritger PL, Peppas NA (1987) A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 5:23–36
Acknowledgements
This work was financially supported by Hunan Provincial Natural Science Foundation of China (Youth Program, No. 2019JJ50584), the PhD Research Startup Foundation of Xiangtan University (No. 18QDZ21), and the Research Foundation of Education Bureau of Hunan Province (No. 18B068), Science Research Foundation of Hunan Provincial Education Department (No. 19C1757).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, C., Wang, L., Xu, Z. et al. Smart micelles self-assembled from four-arm star polymers as potential drug carriers for pH-triggered DOX release. J Polym Res 27, 111 (2020). https://doi.org/10.1007/s10965-020-02108-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-020-02108-2