Abstract
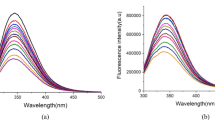
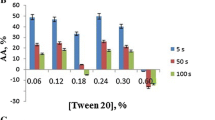
Background: The health benefits of natural products have a long history. Chaga (Inonotus obliques) is used in traditional medicine and is an essential antioxidant for protecting the body from oxidants. Reactive oxygen species (ROS) are produced routinely due to metabolic processes. However, environmental pollution factors such as methyl tert-butyl ether (MTBE) can increase oxidative stress in the human body. MTBE is widely used as a fuel oxygenator that can harm health. The widespread use of MTBE has posed significant threats to the environment by polluting environmental resources, including groundwater. This compound can accumulate in the bloodstream by inhaling polluted air, with a strong affinity for blood proteins. The primary mechanism of MTBE’s harmful effects is ROS production. The use of antioxidants may help reduce MTBE oxidation conditions. The present study proposes that biochaga, as an antioxidant, can reduce MTBE damage in the bovine serum albumin (BSA) structure. Methods and Results: This study investigated the role of different concentrations of biochaga in the structural change of BSA in the presence of MTBE by biophysical methods such as UV-Vis, fluorescence, FTIR spectroscopy, DPPH radical inhibition method, aggregation test, and molecular docking. Research at the molecular level is critical to investigate the structural change of proteins by MTBE and the protective effect of the ideal dose (2.5 µg/ml) of biochaga. Conclusion: the results of spectroscopic examinations showed that the concentration of 2.5 µg/ml of biochaga has the least destructive effect on the structure of BSA in the presence and absence of MTBE, and it can play as an antioxidant.







Similar content being viewed by others
References
Liu Z, Yu D, Li L, Liu X, Zhang H, Sun W, Lin CC, Chen J, Chen Z, Wang W, Jia W (2019) Three-phase partitioning for the extraction and purification of polysaccharides from the immunomodulatory medicinal mushroom Inonotus obliquus. Molecules 24:403. https://doi.org/10.3390/molecules24030403
Song FQ, Liu Y, Kong XS, Chang W, Song G (2013) Progress on understanding the anticancer mechanisms of medicinal mushroom: Inonotus obliquus. Asian Pac J Cancer Prev 14:1571–1578. https://doi.org/10.7314/APJCP.2013.14.3.1571
Géry A, Dubreule C, André V, Rioult JP, Bouchart V, Heutte N, Eldin de Pécoulas P, Krivomaz T, Garon D (2018) Chaga (Inonotus obliquus), a future potential medicinal fungus in oncology? A chemical study and a comparison of the cytotoxicity against human lung adenocarcinoma cells (A549) and human bronchial epithelial cells (BEAS-2B). Integr Cancer Ther 17:832–843. https://doi.org/10.1177/1534735418757912
Szychowski KA, Skóra B, Pomianek T, Gmiński J (2021) Inonotus obliquus–from folk medicine to clinical use. J Tradit Complement Med 11:293–302. https://doi.org/10.1016/j.jtcme.2020.08.003
Szczepkowski A, Pietka J, Grzywacz A (2013) Biology and medicinal properties of the chaga mushroom Inonotus obliquus (Fr.) Pilat. Sylwan 157:223–233
Zheng W, Miao K, Liu Y, Zhao Y, Zhang M, Pan S, Dai Y (2010) Chemical diversity of biologically active metabolites in the sclerotia of Inonotus obliquus and submerged culture strategies for up-regulating their production. Appl Microbiol Biotechnol 87:1237–1254. https://doi.org/10.1007/s00253-010-2682-4
Duru KC, Kovaleva EG, Danilova IG, van der Bijl P (2019) The pharmacological potential and possible molecular mechanisms of action of Inonotus obliquus from preclinical studies. Phytother Res 33:1966–1980. https://doi.org/10.1002/ptr.6384
Taji S, Yamada T, Wada SI, Tokuda H, Sakuma K, Tanaka R (2008) Lanostane-type triterpenoids from the sclerotia of Inonotus obliquus possessing anti-tumor promoting activity. Eur J Med Chem 43:2373–2379. https://doi.org/10.1016/j.ejmech.2008.01.037
Cui Y, Kim DS, Park KC (2005) Antioxidant effect of Inonotus obliquus. J Ethnopharmacol 96:79–85. https://doi.org/10.1016/j.jep.2004.08.037
Staniszewska JUSTYNA, Szymanski M, Ignatowicz E (2017) Antitumor and immunomodulatory activity of Inonotus obliquus. Herba Pol 63:48–58. https://doi.org/10.1515/hepo-2017-0013
Shibnev VA, Mishin DV, Garaev TM, Finogenova NP, Botikov AG, Deryabin PG (2011) Antiviral activity of Inonotus obliquus fungus extract towards infection caused by hepatitis C virus in cell cultures. Bulletin of experimental biology and medicine. Bull Exp Biol Med 151:612–614. https://doi.org/10.1007/s10517-011-1395-8
Ding X, Ge B, Wang M, Zhou H, Sang R, Yu Y, Xu L, Zhang X Inonotus obliquus polysaccharide ameliorates impaired reproductive function caused by Toxoplasma gondii infection in male mice via regulating Nrf2-PI3K/AKT pathway. Int J Biol Macromol 151:449–458., https://doi.org/10.1016/j.ijbiomac.2020.02.178
Han Y, Nan S, Fan J, Chen Q, Zhang Y (2020) (2019) Inonotus obliquus polysaccharides protect against Alzheimer’s disease by regulating Nrf2 signaling and exerting antioxidative and antiapoptotic effects. Int J Biol Macromol 131:769–778. https://doi.org/10.1016/j.ijbiomac.2019.03.033
Živković L, Bajić V, Topalović D, Bruić M, Spremo-Potparević B (2019) Antigenotoxic effects of biochaga and dihydroquercetin (taxifolin) on H2O2-induced DNA damage in human whole blood cells. Oxid Med Cell Longev. https://doi.org/10.1155/2019/5039372
Salimi A, Vaghar-Moussavi M, Seydi E, Pourahmad J (2016) Toxicity of methyl tertiary-butyl ether on human blood lymphocytes. Environ Sci Pollut Res 23:8556–8564. https://doi.org/10.1007/s11356-016-6090-x
Xie G, Hong WX, Zhou L, Yang X, Huang H, Wu D, Huang X, Zhu W, Liu J (2017) An investigation of methyl tert–butyl ether–induced cytotoxicity and protein profile in chinese hamster ovary cells. Mol Med Rep 16:8595–8604. https://doi.org/10.3892/mmr.2017.7761
Hakkola MA, Saarinen LH (2000) Customer exposure to gasoline vapors during refueling at service stations. J Occup Environ Hyg 15:677–680. https://doi.org/10.1080/10473220050110086
Ren Q, Xie X, Tang Y, Hu Q, Du Y (2021) Methyl tertiary-butyl ether inhibits THP-1 macrophage cholesterol efflux in vitro and accelerates atherosclerosis in ApoE-deficient mice in vivo. Res J Environ Sci 101:236–247. https://doi.org/10.1016/j.jes.2020.08.011
Saeedi A, Omidi M, Khoshnoud MJ, Mohammadi-Bardbori A (2015) Exposure to methyl tert-butyl methyl ether (MTBE) is associated with mitochondrial dysfunction in rat. Xenobiotica 47:423–430. https://doi.org/10.3109/00498254.2015.1125040
Fiedler N, Kelly-McNeil K, Mohr S, Lehrer P, Opiekun RE, Lee C, Wainman T, Hamer R, Weisel C, Edelberg R, Lioy PJ (2000) Controlled human exposure to methyl tertiary butyl ether in gasoline: symptoms, psychophysiologic and neurobehavioral responses of self-reported sensitive persons. Environ Health Perspect 108:753–763. https://doi.org/10.1289/ehp.00108753
Bogen KT, Heilman JM (2015) Reassessment of MTBE cancer potency considering modes of action for MTBE and its metabolites. Crit Rev Toxicol 45:1–56. https://doi.org/10.3109/10408444.2015.1052367
Saeedi A, Fardid R, Khoshnoud MJ, Kazemi E, Omidi M, Mohammadi-Bardbori A (2017) Disturbance of zinc and glucose homeostasis by methyl tert-butyl ether (MTBE); evidence for type 2 diabetes. Xenobiotica 47:547–552. https://doi.org/10.1080/00498254.2016.1201872
Valipour M, Maghami P, Habibi-Rezaei M, Sadeghpour M, Khademian MA, Mosavi K, Sheibani N, Moosavi-Movahedi AA (2015) Interaction of insulin with methyl tert-butyl ether promotes molten globule-like state and production of reactive oxygen species. Int J Biol Macromol 80:610–614. https://doi.org/10.1016/j.ijbiomac.2015.07.030
Valipour M, Maghami P, Habibi-Rezaei M, Sadeghpour M, Khademian MA, Mosavi K, Ahmad F, Moosavi-Movahedi AA (2017) Counteraction of the deleterious effects of reactive oxygen species on hemoglobin structure and function by ellagic acid. J Lumin 182:1–7. https://doi.org/10.1016/j.jlumin.2016.10.003
Jahanban-Esfahlan A, Panahi-Azar V, Interaction of glutathione with bovine serum albumin: Spectroscopy and molecular docking. Food chem 202:426–431., Ketrat S, Japrung D, Pongprayoon P (2016) (2020) Exploring how structural and dynamic properties of bovine and canine serum albumins differ from human serum albumin. J Mol Graph 98:107601. https://doi.org/10.1016/j.foodchem.2016.02.026
Ketrat S, Japrung D, Pongprayoon P (2016) (2020) Exploring how structural and dynamic properties of bovine and canine serum albumins differ from human serum albumin. J Mol Graph 98:107601. https://doi.org/10.1016/j.jmgm.2020.107601
Bujacz A (2012) Structures of bovine, equine, and leporine serum albumin. Acta Crystallogr D Biol Crystallogr 68:1278–1289. https://doi.org/10.1107/S0907444912027047
Teng Y, Liu R, Li C, Xia Q, Zhang P (2011) The interaction between 4-aminoantipyrine and bovine serum albumin: multiple spectroscopic and molecular docking investigations. J Hazard Mater 190:574–581. https://doi.org/10.1016/j.jhazmat.2011.03.084
Roufegarinejad L, Jahanban-Esfahlan A, Sajed‐Amin S, Panahi‐Azar V, Tabibiazar M (2018) Molecular interactions of thymol with bovine serum albumin: Spectroscopic and molecular docking studies. J Mol Recognit 31:e2704. https://doi.org/10.1002/jmr.2704
Jahanban-Esfahlan A, Davaran S, Moosavi-Movahedi AA, Dastmalchi S (2017) Investigating the interaction of juglone (5-hydroxy-1, 4-naphthoquinone) with serum albumins using spectroscopic and in silico methods. J Iran Chem Soc 14:1527–1540. https://doi.org/10.1007/s13738-017-1094-0
Jahanban-Esfahlan A, Panahi‐Azar V, Sajedi S (2015) Spectroscopic and molecular docking studies on the interaction between N‐acetyl cysteine and bovine serum albumin. Biopolymers 103:638–645. https://doi.org/10.1002/bip.22697
Roufegarinejad L, Amarowicz R, Jahanban-Esfahlan A (2019) Characterizing the interaction between pyrogallol and human serum albumin by spectroscopic and molecular docking methods. J Biomol Struct Dyn 37:2766–2775. https://doi.org/10.1080/07391102.2018.1496854
Jahanban-Esfahlan A, Dastmalchi S, Davaran S (2016) A simple improved desolvation method for the rapid preparation of albumin nanoparticles. Int J Biol Macromol 91:703–709. https://doi.org/10.1016/j.ijbiomac.2016.05.032
Jahanban-Esfahlan A, Davaran S, Dastmalchi S (2022) Preparation and antiproliferative activity evaluation of juglone-loaded BSA nanoparticles. Adv Pharm Bull 12:818–827. https://doi.org/10.34172/apb.2022.087
Jahanban-Esfahlan A, Ostadrahimi A, Jahanban-Esfahlan R, Roufegarinejad L, Tabibiazar M, Amarowicz R (2019) Recent developments in the detection of bovine serum albumin. Int J Biol Macromol 138:602–617. https://doi.org/10.1016/j.ijbiomac.2019.07.096
Jahanban-Esfahlan A, Roufegarinejad L, Jahanban-Esfahlan R, Tabibiazar M, Amarowicz R (2020) Latest developments in the detection and separation of bovine serum albumin using molecularly imprinted polymers. Talanta 207:120317
Wu S, Wang X, Bao Y, Zhang C, Liu H, Li Z, Chen M, Wang C, Guo Q, Peng X (2020) Molecular insight on the binding of monascin to bovine serum albumin (BSA) and its effect on antioxidant characteristics of monascin. Food chem 315:126228. https://doi.org/10.1016/j.foodchem.2020.126228
Jiao Q, Zhang W, Jiang Y, Jiang L, Chen X, Liu B (2019) Study on the interactions between caffeoylquinic acids with bovine serum albumin: Spectroscopy, antioxidant activity, LC-MSn, and molecular docking approach. Front chem 7:840. https://doi.org/10.3389/fchem.2019.00840
Shi JH, Lou YY, Zhou KL, Pan DQ (2018) Elucidation of intermolecular interaction of bovine serum albumin with Fenhexamid: a biophysical prospect. J Photochem Photobiol B 180:125–133. https://doi.org/10.1016/j.jphotobiol.2018.01.025
Precupas A, Sandu R, Leonties AR, Anghel DF, Popa VT (2017) Complex interaction of caffeic acid with bovine serum albumin: calorimetric, spectroscopic and molecular docking evidence. New J Chem 41:15003–15015. https://doi.org/10.1039/C7NJ03410E
Emadi M, Maghami P, Khorsandi K, Hosseinzadeh R (2019) Biophysical study on the interaction of cartap hydrochloride and hemoglobin: heme degradation and functional changes of protein. J Biochem Mol Toxicol 33:e22325. https://doi.org/10.1002/jbt.22325
Kumari M, Maurya JK, Singh UK, Khan AB, Ali M, Singh P, Patel R (2014) Spectroscopic and docking studies on the interaction between pyrrolidinium based ionic liquid and bovine serum albumin. Spectrochim Acta A Mol Biomol Spectrosc 124:349–356. https://doi.org/10.1016/j.saa.2014.01.012
Shi JH, Pan DQ, Jiang M, Liu TT, Wang Q (2016) Binding interaction of ramipril with bovine serum albumin (BSA): insights from multi-spectroscopy and molecular docking methods. J Photochem Photobiol B 164:103–111. https://doi.org/10.1016/j.jphotobiol.2016.09.025
He Z, Xu M, Zeng M, Qin F, Chen J (2016) Interactions of milk α-and β-casein with malvidin-3-O-glucoside and their effects on the stability of grape skin anthocyanin extracts. Food Chem 199:314–322. https://doi.org/10.1016/j.foodchem.2015.12.035
Shen GF, Liu TT, Wang Q, Jiang M, Shi JH (2015) Spectroscopic and molecular docking studies of binding interaction of gefitinib, lapatinib, and sunitinib with bovine serum albumin (BSA). J Photochem Photobiol B 153:380–390. https://doi.org/10.1016/j.jphotobiol.2015.10.023
Tayyab S, Min LH, Kabir M, Kandandapani S, Ridzwan NFW, Mohamad SB (2020) Exploring the interaction mechanism of a dicarboxamide fungicide, iprodione with bovine serum albumin. Chem Pape 74:1633–1646. https://doi.org/10.1007/s11696-019-01015-1
Zhang G, Wang L, Pan J (2012) Probing the binding of the flavonoid diosmetin to human serum albumin by multispectroscopic techniques. J Agric Food Chem 60:2721–2729. https://doi.org/10.1021/jf205260g
Tan C, Atas E, Müller JG, Pinto MR, Kleiman VD, Schanze KS (2004) Amplified quenching of a conjugated polyelectrolyte by cyanine dyes. J Am Chem Soc 126(42):13685–13694. https://doi.org/10.1021/ja046856b
Benesi HA, Hildebrand JHJ (1949) A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J Am Chem Soc 71:2703–2707. https://doi.org/10.1021/ja01176a030
Goswami S, Sen D, Das NK, Fun HK, Quah CKs (2011) A new rhodamine based colorimetric ‘off–on’fluorescence sensor selective for pd. along with the first bound X-ray crystal structure Chem commun 47:9101–9103. https://doi.org/10.1039/C1CC12845K
Abdollahi K, Ince C, Condict L, Hung A, Kasapis S (2020) Combined spectroscopic and molecular docking study on the pH dependence of molecular interactions between β-lactoglobulin and ferulic acid. Food Hydrocoll 101:105461. https://doi.org/10.1016/j.foodhyd.2019.105461
Tang B, Huang Y, Ma X, Liao X, Wang Q, Xiong X, Li H (2016) Multispectroscopic and docking studies on the binding of chlorogenic acid isomers to human serum albumin: Effects of esteryl position on affinity. Food Chem 212:434–442. https://doi.org/10.1016/j.foodchem.2016.06.007
Lou YY, Zhou KL, Shi JH, Pan DQ (2017) Characterizing the binding interaction of fungicide boscalid with bovine serum albumin (BSA): a spectroscopic study in combination with molecular docking approach. J Photochem Photobiol B 173:589–597. https://doi.org/10.1016/j.jphotobiol.2017.06.037
Shi JH, Zhou KL, Lou YY, Pan DQ (2018) Multi-spectroscopic and molecular modeling approaches to elucidate the binding interaction between bovine serum albumin and darunavir, a HIV protease inhibitor. Spectrochim Acta A Mol Biomol Spectrosc 188:362–371. https://doi.org/10.1016/j.saa.2017.07.040
Kongot M, Reddy DS, Singh V, Patel R, Singhal NK, Kumar A (2020) A manganese (II) complex tethered with S-benzyldithiocarbazate Schiff base: synthesis, characterization, in-vitro therapeutic activity and protein interaction studies. Spectrochim Acta A Mol Biomol Spectrosc 231:118123. https://doi.org/10.1016/j.saa.2020.118123
Zhan F, Ding S, Xie W, Zhu X, Hu J, Gao J, Li B, Chen Y (2020) Towards understanding the interaction of β-lactoglobulin with capsaicin: multi-spectroscopic, thermodynamic, molecular docking and molecular dynamics simulation approaches. Food Hydrocoll 105:105767. https://doi.org/10.1016/j.foodhyd.2020.105767
Siddiqui GA, Siddiqi MK, Khan RH, Naeem A (2018) Probing the binding of phenolic aldehyde vanillin with bovine serum albumin: evidence from spectroscopic and docking approach. Spectrochim Acta A Mol Biomol Spectrosc 203:40–47. https://doi.org/10.1016/j.saa.2018.05.023
Zhao J, Huang L, Sun C, Zhao D, Tang H (2020) Studies on the structure-activity relationship and interaction mechanism of flavonoids and xanthine oxidase through enzyme kinetics, spectroscopy methods and molecular simulations. Food chem 323:126807. https://doi.org/10.1016/j.foodchem.2020.126807
Belaya I, Chikhirzhina E, Polyanichko A (2017) Interaction of DDP with bovine serum albumin facilitates formation of the protein dimers. J Mol Struct 1140:148–153. https://doi.org/10.1016/j.molstruc.2016.12.107
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by NiloofarSepehri, Masoumeh Valipour, Elmira Parchizadeh, and Parvaneh Maghami. The first draft of the manuscript was written by NiloofarSepehri, and all authors commented on previous versions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Statements and Declarations
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Conflict of Interest
The authors have no relevant financial or non-financial interests to disclose.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sepehri, N., Valipour, M., Parchizadeh, E. et al. Investigating the Protective Role of Biochaga Drug on Structural Changes of Bovine Serum Albumin in the Presence of Methyl tert-butyl Ether. Protein J 42, 112–124 (2023). https://doi.org/10.1007/s10930-023-10102-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-023-10102-y