Abstract
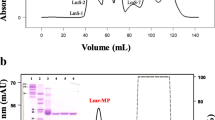
BmHF-1, from the venom of Bothrops marajoensis, was purified by Sephadex G-75 and HPLC-RP on μ-Bondapak C-18 column chromatography. It presented a molecular mass of 27162.36 Da determined by MALDI-TOF MS. BmHF-1 had a sequence of 238 residues of amino acids. The multiple alignment of its amino acid sequence and those of other snake venom metalloproteinases showed high structural similarity, mainly among P–I class. The enzyme initially cleaves the Aα-chain of fibrinogen, followed by the Bβ-chain, and shows no effects on the γ-chain. BmHF-1 had, caseinolytic and weakly hemorrhagic activities, which were inhibited by EDTA. In contrast, PMSF did not affect these activities. The caseinolytic activity of BmHF-1 had a pH optimum of 8.0 and was stable in solution up to 40 °C; activity was completely lost at ≥70 °C. The proteolytic activity was also inhibited by sDa (opossum sera) and Da2-1, Da2-II, antihemorrhagic factors isolated from the opossum sera of Didelphis albiventris. BmHF-1 presents weak hemorrhagic activity, with a MHD of 41.14 μg and it induces dose-dependent edema. We could concluded that, despite its weak hemorrhagic activity, BmHF-1 contributes to local tissue damage by inducing edema, releasing pharmacologically active mediators from protein precursors due to its enzymatic action.





Similar content being viewed by others
Abbreviations
- HPLC-FR:
-
High performance liquid chromatograph reverse phase
- BmHF-1:
-
Metalloproteinase from Bothrops marajoensis snake venom
- MHD:
-
The minimum hemorrhagic dose
- EDTA:
-
Ethylenediamine tetraacetic acid
- EGTA:
-
Ethyleneglycol tetraacetic acid
- PMSF:
-
Phenylmethanesulfonylfluoride or phenylmethylsulfonyl fluoride
- DL-BapNA:
-
The synthetic substrate Na-Benzoyl-d,l-arginine 4-nitroanilide hydrochloride
- DTT:
-
Dithiothreitol
- MALDI-TOF MS:
-
Mass Spectrometry Matrix-assisted laser desorption/ionization (Type: Time of fligth)
- RC-BmHF-1:
-
The reduced carboxymethylated BmHF-1
- CK:
-
The plasma creatine kinase
References
Aguilar I, Girón ME, Rodríguez-Acosta A (2001) Biochim Biophys Acta 1548:57–65
Assakura MT, Reichl AP, Mandelbaum FR (1985) Toxicon 23:691–706
Assakura MT, Reichl AP, Mandelbaum FR (1986) Toxicon 24:943–946
Assakura MT, Silva CA, Mentele R, Camargo AC, Serrano SM (2003) Toxicon 41:217–227
Baker BJ, Wongvibulsin S, Nyborg J, Tu AT (1995) Arch Biochem Biophys 317:357–364
Bello CA, Hermogenes ALN, Magalhaes A, Veiga SS, Gremski LH, Richardson M, Sanchez EF (2006) Biochimie 88:189–200
Bjärnason JB, Fox JW (1994) Pharmacol Ther 62:325–372
Bjärnason JB, Tu AT (1978) Biochemistry 17:3395–3404
Bode W, Gomis-Ruth FX, Stocker W (1993) FEBS Lett 331:134–140
Borkow G, Gutiérrez JM, Ovadia M (1993) Toxicon 31:1137–1150
Braud S, Bon C, Wisne A (2000) Biochimie 82:851–859
Cardoso JLZ, França FOS, Wen F, Malaque CMS, Vidal HJ (2003) Editorial Sarvier São Paulo
Clissa PB, Laing GD, Theakston RD, Mota I, Taylor MJ, Moura-da-Silva AM (2001) Toxicon 39:1567–1573
Costa EP, Clissa PB, Teixeira CFP, Moura-da-Silva AM (2002) Inflammation 26:13–17
Fan HW, Cardoso JL (1995) Handbook of clinical toxicology of animal venoms and poisons. pp 667–688
Fox JW, Serrano SMT (2008) FEBS J 275:3016–3030
Fox JW, Serrano SMT (2005) Toxicon 45:969–985
Franceschi A, Rucavado A, Mora N, Gutiérrez JM (2000) Toxicon 38:63–77
Gomis-Ruth FX, Kress LF, Bode W (1993) EMBO J 12(11):4151–4157
Grams F, Huber R, Kress LF, Moroder L, Bode W (1993) FEBS Lett 335:76–80
Gutiérrez JM, Lomonte B (1995) Toxicon 33:1405–1424
Gutiérrez JM, Rucavado A (2000) Biochimie 82:841–850
Gutiérrez JM, Geni JA, Rojas G, Cerdas L (1985) Toxicon 23:887–893
Gutierrez JM, Romero M, Diaz C, Borkow G, Ovadia M (1995) Toxicon 33:19–29
Gutiérrez JM, Romero M, Núñez J, Chaves F, Borkow G, Ovadia M (1995) Exp Mol Pathol 62:28–41
Gutiérrez JM, Rucavado A, Escalante T, Díaz C (2005) Toxicon 45:997–1011
Heinrikson RL, Meredith SC (1984) Anal Biochem 13:65–72
Hite LA, Jia LG, Bjärnason JB, Fox JW (1994) Arch Biochem Biophys 308:182–191
Kamiguti AS, Zuzel M, Theakston RGD (1998) Braz J Med Biol Res 31:853–862
Khow O, Chanhome L, Omori-Satoh LT, Puempunpanich S, Sitprija V (2002) Toxicon 40:455–461
Laemmli UK (1970) Nature 227:680–685
Laing GD, Clissa PB, Theakston RD, Moura-da-Silva AM, Taylor MJ (2003) Eur J Immunol 33:3458–3463
Leonardi A, Gubenšek F, Križaj I (2002) Toxicon 40:55–62
Lewis RJ, Garcia ML (2003) Nat Rev Drug Discov 2:790–802
López-Lozano JL, de Sousa MV, Ricart CA, Chávez-Olortegui C, Sanchez E, Muniz EG, Bührnheim PF, Morhy L (2002) Toxicon 40:997–1006
Mandelbaum FR, Reichel AP, Assakura MT (1982) Toxicon 20:955–972
Marcussi S, Bernardes CP, Santos-Filho NA, Mazzi MV, Oliveira CZ, Izidoro LFM, Fuly AL, Magro AJ, Braz ASK, Fontes MRM, Giglio JG, Soares AM (2007) Peptides 28:2328–2339
Marsh NA (2001) Haemostasis 31:211–217
Maruyama M, Sugiki M, Anai K, Yoshida E (2002) Toxicon 40:1223–1226
Maruyama M, Tanigawa M, Sugiki M, Yoshida E, Mihara H (1993) Enzyme Protein 47:124–135
Mazzi MV, Marcussi S, Carlos G, Stábeli RG, Franco JJ, Ticli FK, Cintra AC, França SC, Soares AM, Sampaio SV (2004) Toxicon 44:215–223
Ohsaka A, Just M, Habermann E (1973) Biochim Biophys Acta 323:415–428
Ownby CL, Colberg TR, Li Q (1994) Toxicon 32:945–954
Peichoto ME, Teibler P, Mackessy SP, Leiva L, Acosta O, Gonçalves LRC, Tanaka-Azevedo AM, Santoro ML (2007) Biochim Biophys Acta 1770:810–819
Petretski JH, Kanashiro MM, Rodrigues FR, Alves EW, Machado OL, Kipnis TL (2000) Biochem Biophys Res Commun 276:29–34
Ponce-Soto LA, Baldasso PA, Romero-Vargas FF, Winck FV, Novello JC, Marangoni S (2007) Protein J 26:39–49
Ramos OHP, Selistre-de-Araujo HS (2006) Comp Biochem Physiol C Toxicol Pharmacol 142:328–346
Raquel KB, Murakami MT, Watanabe L, Soares AM, Arni RK (2002) Toxicon 40:1307–1312
Rodrigues VM, Soares AM, Guerra-Sa R, Rodrigues V, Fontes R, Giglio JR (2000) Arch Biochem Biophys 381(2):213–224
Rosenfeld G (1971) In: Bucherl W, Buckley EE (eds) Venomous animals and their venoms, vol 2. Academic Press, New York, pp 345–403
Rucavado A, Escalante T, Teixeira CF, Fernándes CM, Diaz C, Gutiérrez JM (2002) Mediators Inflamm 11:121–128
Rucavado A, Flores-Sánchez E, Franceschi A, Magalhaes A, Gutiérrez JM (1999) Toxicon 37:1297–1312
Rucavado A, Núñez J, Gutiérrez JM (1998) Int J Exp Pathol 79:245–254
Sanchez EF, Diniz CR, Richardson M (1991) FEBS Lett 282:178–182
Takeya H, Onikura A, Nikai T, Sugihara H, Iwanaga S (1990) J Biochem 108:711–719
Watanabe L, Shannon JD, Valente RH, Rucavado A, Alape-Girón A, Kamiguti AS, Theakston RD, Fox JW, Gutiérrez JM, Arni RK (2003) Protein Sci 12:2273–2281
Acknowledgments
The authors thank Mr. Paulo A. Baldasso for technical assistance. This work was supported by the National Council for Scientific and Technological Development (CNPq) and FAPESP process 06/51566-0. It is part of Frank Denis Torres-Huaco Mg thesis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Torres-Huaco, F.D., Ponce-Soto, L.A., Martins-de-Souza, D. et al. Purification and Characterization of a New Weak Hemorrhagic Metalloproteinase BmHF-1 from Bothrops marajoensis Snake Venom. Protein J 29, 407–416 (2010). https://doi.org/10.1007/s10930-010-9267-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-010-9267-z