Abstract
In this study, the nanohybrid drug carrier were synthesized by Pickering emulsion-templated encapsulation (PETE) method to control the sustained-released properties of the nanohybrid drug carrier; magadiite-cetyltriphenyl phosphonium bromide (MAG-CTPB-KH550) and sodium alginate (NaC6H7O6) was dissolved in the aqueous phase but metronidazole (C6H9N3O3) was dissolved in the ethyl acetate (CH3COOC2H5) of the oil phase; both the oil phase and the aqueous phase were mixed and dispersed to prepared organically-modified magadiite-sodium alginate (MAG–CTPB–KH550/SA) nanohybrid drug carrier. X-ray diffraction (XRD), Flourier transform infrared spectrometry (FTIR) and scanning electron microscopy (SEM) results were shown that the most of Sodium alginate (SA) were encapsulated into the MAG–CTPB–KH550 but a few of SA were intercalated into the inner space layers of MAG–CTPB–KH550, metronidazole was combined with carrier materials through physical apparent adsorption, ion exchange and electrostatic interaction. In vitro result, it was showed that the slow release was shown less than 10% content of Sodium alginate; whereas, it was reduced the initial release percentage of Metronidazole but it was extended the sustained-released time. To reach at equilibrium, the sustained-released effects of the drug carrier were prepared with 10% of Sodium Alginate for 32 h and the maximum cumulative release percentage was 93.42% for 24 h. First order model, Baker–Lonsdale model and Korsmeyer–Peppas model were fitted to study the slow-release mechanism; the correlation coefficients (R2) of the three models were found over 0.9; thus, it was well described the release kinetics behavior of drug carrier composites. The slow-release mechanisms of the drug carrier were performed swelling and dissolving but the barrier effects of the lamina that were reduced the dissolution percentage of metronidazole.
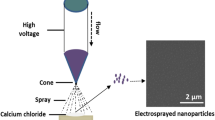
Graphical Abstract







Similar content being viewed by others
Data Availability
Not Applicable.
References
Yang J, Chen J, Pan D, Wan Y, Wang Z (2013) pH-sensitive interpenetrating network hydrogels based on chitosan derivatives and alginate for oral drug delivery. Carbohydr Polym 92:719–725
Eugster HP (1967) Hydrous sodium silicate from Lake Magadi, Kenya, precursors of bedded chert. Science 157:1177–1179
Allen TM, Cullis PR (2013) Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev 65:36–48
Pal K, Laha D, Parida PK, Roy S, Bardhan S, Dutta A et al (2019) An in vivo study for targeted delivery of curcumin in human triple negative breast carcinoma cells using biocompatible PLGA microspheres conjugated with folic acid. J Nanosci Nanotechnol 19:3720–3733
Ling K, Wu H, Neish AS, Champion JA (2019) Alginate/chitosan microparticles for gastric passage and intestinal release of therapeutic protein nanoparticles. J Control Release 295:174–186
Guo R, Sun X-T, Zhang Y, Wang D-N, Yang C-G, Xu Z-R (2018) Three-dimensional poly(lactic-co-glycolic acid)/silica colloidal crystal microparticles for sustained drug release and visualized monitoring. J Colloid Interface Sc 530:465–472
Porgham D, Dusti T, Hossein K, Akbarijavar H, Khoobi M, Seyedjafari E, Birhanu G, Khosravian P, SadatMahdavi F (2019) Poly-l-lactic acid scaffold incorporated chitosan-coated mesoporous silica nanoparticles as pH-sensitive composite for enhanced osteogenic differentiation of human adipose tissue stem cells by dexamethasone delivery. Artif Cells Nanomed Biotechnol 47:4020–4029
Tang F, Li L, Chen D (2012) Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater 24:1504–1534
Mehrzad-Samarin M, Faridbod F, Dezfuli AS, Ganjali MR (2017) A novel metronidazole fluorescent nanosensor based on graphene quantum dots embedded silica molecularly imprinted polymer. Biosens Bioelectron 92:618–623
Pawar SN, Edgar KJ (2012) Alginate derivatization: a review of chemistry, properties and applications. Biomaterials 33:3279–3305
Zhang Y, Wei W, Lv P, Wang L, Ma G (2011) Preparation and evaluation of alginate–chitosan microspheres for oral delivery of insulin. Eur J Pharm Biopharm 77:11–19
Sarmento B, Ribeiro A, Veiga F, Sampaio P, Neufeld R, Ferreira D (2007) Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm Res 24:2198–2206
Mukhopadhyay P, Chakraborty S, Bhattacharya S, Mishra R, Kundu PP (2015) pH-sensitive chitosan/alginate core-shell nanoparticles for efficient and safe oral insulin delivery. Int J Biol Macromol 72:640–648
Ribeiro AJ, Silva C, Ferreira D, Veiga F (2005) Chitosan-reinforced alginate microspheres obtained through the emulsification/internal gelation technique. Eur J Pharm Sci 25:31–40
Chávarri M, Marañón I, Ares R, Ibáñez FC, Marzo F, Villarán M (2010) Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int J Food Microbiol 142:185–189
Cook MT, Tzortzis G, Charalampopoulos D, Khutoryanskiy VV (2011) Production and evaluation of dry alginate-chitosan microcapsules as an enteric delivery vehicle for probiotic bacteria. Biomacromol 12:2834–2840
Tan W-H, Takeuchi S (2007) Monodisperse alginate hydrogel microbeads for cell encapsulation. Adv Mater 19:2696–2701
Tsirigotis-Maniecka M, Gancarz R, Wilk KA (2016) Preparation and characterization of sodium alginate/chitosan microparticles containing esculin. Colloids Surf A 510:22–32
Liu P, Krishnan TR (1999) Alginate-pectin-poly-L-lysine particulate as a potential controlled release formulation. J Pharm Pharmacol 51:141–149
Kulkarni AR, Soppimath KS, Aralaguppi MI, Aminabhavi TM, Rudzinski WE (2000) Preparation of cross-linked sodium alginate microparticles using glutaraldehyde in methanol. Drug Dev Ind Pharm 26:1121–1124
Kulkarni AR, Soppimath KS, Aminabhavi TM, Dave AM, Mehta MH (2000) Glutaraldehyde crosslinked sodium alginate beads containing liquid pesticide for soil application. J Control Release 63:97–105
Bardajee GR, Hooshyar Z (2018) Thermo/pH/magnetic-triple sensitive poly(N-isopropylacrylamide-co-2-dimethylaminoethyl) methacrylate)/sodium alginate modified magnetic graphene oxide nanogel for anticancer drug delivery. Polym Bull 75:5403–5419
Xie C-X, Tian T-C, Yu S-T, Li L (2019) pH-sensitive hydrogel based on carboxymethyl chitosan/sodium alginate and its application for drug delivery. J Appl Polym Sci 136:46911
Yan H, Chen X, Feng Y, Xiang F, Li J, Shi Z et al (2016) Modification of montmorillonite by ball-milling method for immobilization and delivery of acetamiprid based on alginate/exfoliated montmorillonite nanocomposite. Polym Bull 73:1185–1206
Iliescu RI, Andronescu E, Ghitulica CD, Voicu G, Ficai A, Hoteteu M (2014) Montmorillonite–alginate nanocomposite as a drug delivery system—incorporation and in vitro release of irinotecan. Int J Pharm 463:184–192
Mokhtar A, Djelad A, Adjdir M, Zahraoui M, Bengueddach A, Sassi M (2018) Intercalation of hydrophilic antibiotic into the interlayer space of the layered silicate magadiite. J Mol Struct 1171:190–195
Homhuan N, Bureekaew S, Ogawa M (2017) Efficient concentration of Indium(III) from aqueous solution using layered silicates. Langmuir 33:9558–9564
Auerbach SM, Carrado KA, Dutta PK (2004) Handbook of layered materials. CRC Press, Boca Raton
Ge M, Cao L, Du M, Hu G, Jahangir Alam SM (2018) Competitive adsorption analyses of a pure magadiite and a new silylated magadiite on methylene blue and phenol from related aqueous solution. Mater Chem Phys 217:393–402
Ge M, Wang X, Du M, Liang G, Hu G et al (2019) Adsorption analyses of phenol from aqueous solutions using magadiite modified with organo-functional groups: kinetic and equilibrium studies. Materials 12:96
Pathak C P, Sawhney A S, Edelman P G, Chandrashekhar, Bennett S (2000) Biocompatible crosslinked polymer preparation by nucleophilic-electrophilic group reaction between functional polymer and crosslinking agent, useful e.g. for preventing surgical adhesions or for drug delivery: WO 200033764-A1[P] 6–15.
Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE (2001) Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release 70:1–20
Khodaverdi E, Tayarani-Najaran Z, Minbashi E, Alibolandi M, Hosseini J, Sepahi S et al (2019) Docetaxel-loaded mixed micelles and polymersomes composed of poly (caprolactone)-poly (ethylene glycol) (PEG-PCL) and poly (lactic acid)-poly (ethylene glycol) (PEG-PLA): preparation and in-vitro characterization. Iran J Pharm Res 18:142–155
Aveyard R, Binks BP, Clint JH (2003) Emulsions stabilised solely by colloidal particles. Adv Coll Interface Sci 100–102:503–546
Low LE, Tan LTH, Goh B-H, Tey BT, Ong BH, Tang SY (2019) Magnetic cellulose nanocrystal stabilized Pickering emulsions for enhanced bioactive release and human colon cancer therapy. Int J Biol Macromol 127:76–84
Wang X, Yu K, An R, Han L, Zhang Y, Shi L et al (2019) Self-assembling GO/modified HEC hybrid stabilized pickering emulsions and template polymerization for biomedical hydrogels. Carbohyd Polym 207:694–703
Javanbakht S, Shaabani A (2019) Encapsulation of graphene quantum dot-crosslinked chitosan by carboxymethylcellulose hydrogel beads as a pH-responsive bio-nanocomposite for the oral delivery agent. Int J Biol Macromol 123:389–397
Goenka S, Sant V, Sant S (2014) Graphene-based nanomaterials for drug delivery and tissue engineering. J Control Release 173:75–88
Marku D, Wahlgren M, Rayner M, Sjöö M, Timgren A (2012) Characterization of starch Pickering emulsions for potential applications in topical formulations. Int J Pharm 428:1–7
Akhavan O, Ghaderi E, Aghayee S, Fereydooni Y, Talebi A (2012) The use of a glucose-reduced graphene oxide suspension for photothermal cancer therapy. J Mater Chem 22:13773–13781
Ge M, Xi Z, Zhu C, Liang G, Hu G, Jamal L et al (2019) Preparation and characterization of magadiite-magnetite nanocomposite with its sorption performance analyses on removal of methylene blue from aqueous solutions. Polymers 11:607
Yin G, Zheng Z, Wang H, Du Q (2011) Slightly surface-functionalized polystyrene microspheres prepared via Pickering emulsion polymerization using for electrophoretic displays. J Colloid Interface Sci 361:456–464
Ge M, Cao L, Du M, Hu G, Alam SMJ (2018) Adsorptive characterization of a pure magadiite and an organic modified magadiite on removal of methylene blue from related aqueous solution. Mater Chem Phys 217:533–540
Barthelat F (2010) Nacre from mollusk shells: a model for high-performance structural materials. Bioinspir Biomim 5:035001
Tang H, Zhang X (2005) Identification of metronidazole tablets by infrared spectrophotometry. China Pharmacy 16(8):619–620
Ge M, Xi Z, Zhu C, Liang G, Hu G, Jamal L et al (2019) Preparation and characterization of magadiite–magnetite nanocomposite with its sorption performance analyses on removal of methylene blue from aqueous solutions. Polymers 11(4):607
Mandapalli PK, Venuganti VVK (2015) Layer-by-layer microcapsules for pH-controlled delivery of small molecules. J Pharm Investig 45:131–141
Ding A, Zhou Y, Chen P, Nie W (2017) Ibuprofen-loaded micelles based on star-shaped erythritol-core PLLA-PEG copolymer: effect of molecular weights of PEG. Colloid Polym Sci 295:1609–1619
Ge M, Tang W, Du M, Liang G, Hu G, Jahangir Alam SM (2019) Research on 5-fluorouracil as a drug carrier material with its in vitro release properties on organic modified magadiite. Eur J Pharm Sci 130:44–53
Nhavene EPF, da Silva WM, Trivelato Junior RR, Gastelois PL, Venâncio T, Nascimento R et al (2018) Chitosan grafted into mesoporous silica nanoparticles as benznidazol carrier for Chagas diseases treatment 272:265–275
Huanbutta K, Nernplod T, Akkaramongkolporn P, Sriamornsak P (2017) Design of porous Eudragit® L beads for floating drug delivery by wax removal technique. Asian J Pharm Sci 12:227–234
Mingliang G, Yueying L, Caiping Z, Guodong L, Jahangir A.S.M., Guoqing H, Yuee G, Junaebur RM (2021) Preparation of organic-modified magadiite–magnetic nanocomposite particles as an effective nanohybrid drug carrier material for cancer treatment and its properties of sustained release mechanism by Korsmeyer–Peppas kinetic model. J Mater Sci 1–17.
Acknowledgements
The authors gratefully acknowledge the financial support of this research work by the Natural Science Foundation of Guangdong Province Project (Project No. 2016A030313520), Key Laboratory of Polymeric Composite & Functional Materials of Ministry of Education Project (Project No. PCFM-2017-02), Guangdong Water Conservancy Science and Technology Innovation Project (Project No. 2017-24), Science and Technology Program of Guangzhou in China (Project No. 202102080477), and Guangdong Provincial Department of Education Featured Innovation Project (Project No. 2017KTSCX007). The authors gratefully thank the reviewers for their valuable review comments to enrich the publication.
Funding
The authors gratefully acknowledge the financial support of this research work by Natural Science Foundation of Guangdong Province Project (Project No. 2016A030313520), Key Laboratory of Polymeric Composite & Functional Materials of Ministry of Education Project (Project No. PCFM-2017-02), Guangdong Water Conservancy Science and Technology Innovation Project (Project No. 2017-24), Science and Technology Program of Guangzhou in China (Project No. 202102080477), and Guangdong Provincial Department of Education Featured Innovation Project (Project No. 2017KTSCX007).
Author information
Authors and Affiliations
Contributions
Conceptualization, MG, YG and GL; Data curation, SMJA, YH, LC and GL; Formal analysis, SMJA, XL, YL, and YH; Funding acquisition, MG; Investigation, MG, XL, YL and YG; Methodology, MG, XL, YL, SMJA, YG, YH, LC and GL; Project administration, MG and GH; Resources, MG, YG, GL and GH; Software, SMJA and YH; Supervision, MG; Validation, MG, XL, YL, YG, LC and GL; Visualization, XL, YL, SMJA, LC; Writing—original draft, YL; Writing—review and editing, SMJA, and GH.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Ethical Approval
Not Applicable.
Consent to Participate
Not Applicable.
Consent for Publication
All the authors are agreed for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ge, M., Li, X., Li, Y. et al. Preparation of Magadiite-Sodium Alginate Drug Carrier Composite by Pickering-Emulsion-Templated-Encapsulation Method and Its Properties of Sustained Release Mechanism by Baker–Lonsdale and Korsmeyer–Peppas Model. J Polym Environ 30, 3890–3900 (2022). https://doi.org/10.1007/s10924-022-02426-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02426-0