Abstract
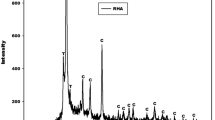
This work aims to investigate the coexistence of the poly(phospho-siloxo) networks and calcium phosphates on the compressive strengths of the acid-based geopolymers obtained at room temperature. Waste fired brick and phosphoric acid were used as an aluminosilicate and chemical reagent, respectively. Calcium aluminate hydrate was prepared by mixing calcium hydroxide from the calcined eggshell and calcined bauxite. Calcium silicate hydrate was obtained by the mixture of rice husk ash and calcium hydroxide. The molar ratios CaO/Al2O3 and CaO/SiO2 in the calcium aluminate and calcium silicate hydrates are equals to 1.0. The X-ray patterns of the acid-based geopolymers indicate the broad hump structure between 18 and 38°(2θ). In addition to this broad band, those from the mixture of calcium sources show the reflection peaks of monetite and brushite. The compressive strength of the reference is 56.43 MPa. Those obtained with the addition of 10, 20, 40 and 50 g of calcined eggshell are 30.15, 22.85, 21.16 and 13.47 MPa, respectively. The ones from calcium aluminate hydrate are 32.62, 31.58, 17.83 and 16.33 MPa, respectively. Whereas those containing calcium silicate hydrate are 44.02, 42.71, 40.19 and 18.59 MPa, respectively. This work demonstrates that the formation of calcium phosphates in the structure of the acid-based geopolymers decreases the poly(phospho-siloxo) chains and therefore reduces their compressive strengths. The moderate addition of calcium silicate hydrate reduces slightly the compressive strengths of the acid-based geopolymers which can be comparable to the one of CEM II 42.5R.


















Similar content being viewed by others
References
C.Y. Heah, H. Kamarudin, A.M.M. Al Bakri, M. Luqman, I. Khairul Nizar, Y.M. Liew, Potential application of kaolin without calcined as greener concrete: a review. Aust. J. Basic Appl. Sci. 5, 1026–1035 (2011)
E. Kamseu, C. Leonelli, D.S. Perera, U.F.C. Melo, P.N. Lemougna, Investigation of volcanic ash-based geopolymers as potential building materials. Int. Ceram. 58, 136–140 (2009)
D. Bondar, C.J. Lynsdale, N.B. Milestone, N. Hassani, A.A. Ramezanianpour, Effect of heat treatment on reactivity-strength of alkali-activated natural pozzolans. Constr. Build. Mater. 25, 4065–4071 (2011)
D. Bondar, C.J. Lynsdale, N.B. Milestone, N. Hassani, A.A. Ramezanianpour, Effect of adding mineral additives to alkali-activated natural pozzolan paste. Constr. Build. Mater. 25, 2906–2910 (2011)
P.N. Lemougna, K.J.D. Mackenzie, U.F.C. Melo, Synthesis and thermal properties of inorganic polymers (geopolymers) for structure and refractory applications from volcanic ash. Ceram. Int. 37, 3011–3018 (2011)
H.K. Tchakouté, A. Elimbi, E. Yanne, C.N. Djangang, Utilization of volcanic ashes for the production of geopolymers cured at ambient temperature. Cem. Concr. Comp. 38, 75–81 (2013)
W.K.W. Lee, J.S.J. van Deventer, Structural reorganisation of class F fly ash in alkaline solutions. Colloids Surf. A 211, 49–66 (2002)
X. Guo, H. Shi, W.A. Dick, Compressive strength and microstructural characteristics of class C fly ash geopolymer. Cem. Concr. Comp. 32, 142–147 (2010)
T.W. Cheng, J.P. Chiu, Fire resistant geopolymer produced by granulated blast furnace slag. Miner. Eng. 16, 205–210 (2003)
A. Elimbi, H.K. Tchakoute, D. Njopwouo, Effects of calcination temperature of kaolinite clays on the properties of geopolymers cements. Constr. Build. Mater. 25, 2805–2812 (2011)
C.K. Yip, G.C. Lukey, J.L. Provis, J.S.J. van Deventer, Effect of calcium silicate sources on geopolymerization. Cem. Concr. Res. 38, 554–564 (2008)
C.K. Yip, G.C. Lukey, J.S.J. van Deventer, The coexistence of geopolymeric gel and calcium silicate hydrate at the early stage of alkaline activation. Cem. Concr. Res. 35, 1688–1697 (2005)
D.E.T. Mabah, H.K. Tchakouté, C.H. Rüscher, E. Kamseu, A. Elimbi, C. Leonelli, Design of low-cost semi-crystalline calcium silicate from biomass for the improvement of the mechanical and microstructural properties of metakaolin-based geopolymer cements. Mater. Chem. Phys. 223, 98–108 (2019)
H.K. Tchakouté, D.E.T. Mabah, C.H. Rüscher, E. Kamseu, F. Andreola, M.C. Bignozzi, C. Leonelli, Preparation of low-cost nano and microcomposites from chicken eggshell, nano-silica and rice husk ash and their utilisations as additives for producing geopolymer cements. J. Asian Ceram. Soc. 8, 149–161 (2020)
K.J.D. MacKenzie, M.E. Smith, A. Wong, A multinuclear MAS NMR study of calcium-containing aluminosilicate inorganic polymers. J. Mater. Chem. 17, 5090–5096 (2007)
F.M. Tchuenté, H.K. Tchakouté, C. Banenzoué, C.H. Rüscher, E. Kamseu, F. Andreola, C. Leonelli, Microstructural and mechanical properties of (Ca, Na)-poly(sialate-siloxo) from metakaolin as aluminosilicate and calcium silicate from precipitated silica and calcined chicken eggshell. Constr. Build. Mater. 201, 662–675 (2019)
H.K. Tchakouté, D. Fotio, C.H. Rüscher, E. Kamseu, J.N.Y. Djobo, M.C. Bignozzi, C. Leonelli, The effects of synthesized calcium phosphate compounds on the mechanical and microstructural properties of metakaolin-based geopolymer cements. Constr. Build. Mater. 163, 776–792 (2018)
M.L. Gualtieri, M. Romagnoli, A.F. Gualtieri, Preparation of phosphoric acid-based geopolymer foams using limestone as pore forming agent-thermal properties by in situ XRPD and Rietveld refinements. J. Eur. Ceram. Soc. 35, 3167–3178 (2015)
N. Boutaleb, F. Chouli, A. Benyoucef, F.Z. Zeggai, K. Bachari, A comparative study on surfactant cetyltrimethylammoniumbromide modified clay-based poly(p-anisidine) nanocomposites: synthesis, characterization, optical and electrochemical properties. Polym. Compos. (2021). https://doi.org/10.1002/pc.25941
D. Cao, D. Su, B. Lu, Y. Yang, Synthesis and structure characterization of geopolymeric material based on metakaolinite and phosphoric acid. J. Chin. Ceram. Soc. 33, 1385–1389 (2005)
D.S. Perera, J.V. Hanna, J. Davis, The relative strength of phosphoric acid-reacted and alkali-reacted metakaolin materials. J. Mater. Sci. 43, 6562–6566 (2008)
H. Celerier, J. Jouin, V. Mathivet, N. Tessier-Doyen, S. Rossignol, Composition and properties of phosphoric acid-based geopolymers. J. Non-Cryst. Solids 493, 94–98 (2018)
M.L. Gualtieri, M. Romagnoli, S. Pollastri, A.F. Gualtieri, Inorganic polymers from laterite using activation with phosphoric acid and alkaline sodium silicate solution: mechanical and microstructural properties. Cem. Concr. Res. 67, 259–270 (2015)
C.N. Bewa, H.K. Tchakouté, C.H. Rüscher, E. Kamseu, C. Leonelli, Influence of the curing temperature on the properties of poly(phospho-ferro-siloxo) networks from laterite. SN Appl. Sci. 1, 1–12 (2019)
J. Davidovits, Geopolymer Chemistry and Applications, 3rd edn. (Institute Geopolymer, Saint-Quentin, 2011), p. 612
C.N. Bewa, H.K. Tchakouté, C. Banenzoué, L. Cakanou, T.T. Mbakop, E. Kamseu, C.H. Rüscher, Acid-based geopolymers using waste fired brick and different metakaolins as raw materials. Appl. Clay Sci. 198, 105813 (2020)
A.B. Tchamba, R. Yongue, U.F.C. Melo, E. Kamseu, D. Njoya, D. Njopwouo, Caractérisation de la bauxite de Haléo-Danielle (Minim-Martap, Cameroun) en vue de son utilisation industrielle dans les matériaux à haute teneur en alumine. Silic. Ind. 73, 77–83 (2008)
D. Prasetyoko, Z. Ramli, S. Endud, H. Hamdan, B. Sulikowski, Conversion of rice husk ash to zeolite beta. Waste Manag. 26, 1173–1179 (2006)
H.K. Tchakouté, C.H. Rüscher, J.N.Y. Djobo, B.B.D. Kenne, D. Njopwouo, Influence of gibbsite and quartz in kaolin on the properties of metakaolin-based geopolymer cements. Appl. Clay Sci. 107, 188–194 (2015)
R.L. Frost, J.T. Kloprogge, S.C. Russell, J.L. Szetu, Vibrational spectroscopy and dehydroxylation of aluminum (oxo)hydroxides: gibbsite. Appl. Spectrosc. 53, 423–434 (1999)
R.L. Frost, J.T. Kloprogge, S.C. Russell, J.L. Szetu, Dehydroxylation and the vibrational spectroscopy of aluminum (oxo)hydroxides using infrared emission spectroscopy. Part III: diaspore. Appl. Spectrosc. 53, 829–835 (1999)
A. Boumaza, L. Favaro, J. Lédion, G. Sattonnay, J.B. Brubach, P. Berthet, A.M. Hunt, P. Roy, R. Tétot, Transition alumina phases induced by heat treatment of boehmite: an X-ray diffraction and infrared spectroscopy study. J. Solid State Chem. 182, 1171–1176 (2009)
X. Pan, D. Zhang, Y. Wu, H. Yu, Synthesis and characterization of calcium aluminate compound from gehlenite by high-temperature solid state reaction. Ceram. Int. 44, 13544–13550 (2018)
Z. Zidi, M. Ltifi, Z. Ben Ayadi, L. ElMir, Synthesis of nano-alumina and their effect on structure, mechanical and thermal properties of geopolymer. J. Asian Ceram. Soc. 7, 524–535 (2019)
K. Baltakys, R. Jauberthie, R. Siauciunas, R. Kaminskas, Influence of modification of SiO2 on the formation of calcium silicate hydrate. Mater. Sci. Poland 25, 663–670 (2007)
C.N. Bewa, H.K. Tchakouté, D. Fotio, C.H. Rüscher, E. Kamseu, C. Leonelli, Water resistance and thermal behaviour of metakaolin-phosphate-based geopolymer cements. J. Asian Ceram. Soc. 6, 271–283 (2018)
B. Zhang, H. Guo, P. Yuan, L. Deng, X. Zhong, Y. Li, Q. Wang, D. Liu, Novel acid-based geopolymer synthesized from nanosized tubular halloysite: the role of precalcination temperature and phosphoric acid concentration. Cem. Concr. Comp. 110, 103601 (2020)
J. Wang, H. Zhao, S. Zhou, X. Lu, B. Feng, C. Duan, J. Weng, One-step in situ synthesis and characterization of sponge-like porous calcium phosphate scaffolds using a sol-gel and gel casting hybrid process. J. Biomedical Mater. Res. Part A 90A, 401–410 (2009)
R. Štulajterová, L. Medvecky, Effect of calcium ions on transformation brushite to hydroxyapatite in aqueous solutions. Colloids Surf. A 316, 104–109 (2008)
M.M. Mirkovic, T.D.L. Pašti, A.M. Došen, M.Z. Cebela, A.A. Rosic, B.Z. Matovic, B.M. Babic, Adsorption of malathion on mesoporous monetite obtained by mechanochemical treatment of brushite. RSC Adv. 6, 12219–12225 (2016)
C.K. Yip, The role of calcium in geopolymerization, University of Melbourne, Australia, PhD Thesis (2004)
A. Moshiri, D. Stefaniuk, S.K. Smith, A. Morshedifard, D.F. Rodrigues, M.J.A. Qomi, K.J. Krakowiak, Structure and morphology of calcium-silicate-hydrates cross-linked with dipodal organosilanes. Cem. Concr. Res. 133, 106076 (2020)
Acknowledgements
Dr. Tchakouté Kouamo Hervé gratefully acknowledges the Alexander von Humboldt-Stiftung for its financial support this work under Grant N° KAM/1155741 GFHERMES-P. The authors would like to thank Dr. Valerie Petrov for SEM observations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Riyap, H.I., Tazune, F.K., Fotio, D. et al. The Coexistence of the Poly(phospho-siloxo) Networks and Calcium Phosphates on the Compressive Strengths of the Acid-Based Geopolymers Obtained at Room Temperature. J Inorg Organomet Polym 31, 3301–3323 (2021). https://doi.org/10.1007/s10904-021-01949-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-021-01949-8