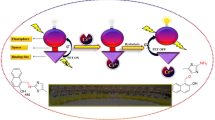
Abstract
In this study, we have synthesized a novel Schiff base-centered chemosensor, designated as SB, with the chemical name ((E)-1-(((6-methylbenzo[d]thiazol-2-yl) imino)methyl)naphthalen-2-ol). This chemosensor was structurally characterized by FT-IR, 1H NMR, UV-Vis and fluorescence spectroscopy. After structural characterization the chemosensor SB was subsequently employed for the detection of Cu2+ and Ag+, using fluorescence spectroscopy. The chemosensor SB showed excellent ability to recognize the target metal ions, leading to fluorescence enhancement and color change from yellow to yellowish orange for Cu2+ and yellow to radish for Ag+ ions. The detection capabilities of this chemosensor were impressive, showing excellent selectivity and an exceptionally low detection limit of 0.0016 µM for Cu2+ and 0.00389 µM for Ag+. Most notably, our approach enables the quantitative detection both metal ions in different water and soil samples at trace level. This achievement holds great promise for analytical applications and offers significant contributions to the field of chemical sensing and environmental protection.




















Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
Code Availability
Chem Draw.
References
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. EXS 101:133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6. https://doi.org/10.1016/j.heliyon.2020.e04691
Wani AL, Ara A, Usmani JA (2015) Lead toxicity: A review. Interdiscip Toxicol 8:55–64. https://doi.org/10.1515/intox-2015-0009
Liu D, Shi Q, Liu C, Sun Q, Zeng X (2023) Effects of endocrine-disrupting heavy metals on human health. Toxics 11. https://doi.org/10.3390/TOXICS11040322
Buccolieri A, Buccolieri G, Dell’Atti A, Perrone MR, Turnone A (2006) Natural sources and heavy metals. Ann Chim 96:167–181. https://doi.org/10.1002/ADIC.200690017
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: A review of sources chemistry, risks and best available strategies for remediation. ISRN Ecol 2011:1–20. https://doi.org/10.5402/2011/402647
Gan Y, Huang X, Li S, Liu N, Li YC, Freidenreich A, Wang W, Wang R, Dai J (2019) Source quantification and potential risk of mercury, cadmium, arsenic, lead, and chromium in farmland soils of Yellow River Delta. J Clean Prod 221:98–107. https://doi.org/10.1016/J.JCLEPRO.2019.02.157
Kulkarni SJ (2020) Heavy metal pollution: Sources, effects, and control methods, hazard. Waste Manag Heal Risks 97–112. https://doi.org/10.2174/9789811454745120010008
Sharma N, Sodhi KK, Kumar M, Singh DK (2021) Heavy metal pollution: Insights into chromium eco-toxicity and recent advancement in its remediation. Environ Nanotechnol Monit Manag 15:100388. https://doi.org/10.1016/j.enmm.2020.100388
Yang Q, Li Z, Lu X, Duan Q, Huang L, Bi J (2018) A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment
Nagajyoti PC, Lee KD, Sreekanth TV (2010) Heavy metals, occurrence and toxicity for plants: A review. Environ Chem Lett 83(8):199–216. https://doi.org/10.1007/S10311-010-0297-8
Peter ALJ, Viraraghavan T (2005) Thallium: A review of public health and environmental concerns. Environ Int 31:493–501. https://doi.org/10.1016/j.envint.2004.09.003
Mohammed AS, Kapri A, Goel R (2011) Heavy metal pollution: Source, impact, and remedies. 1–28. https://doi.org/10.1007/978-94-007-1914-9_1
Fu Z, Xi S (2019) The effects of heavy metals on human metabolism. 30:167–176.https://doi.org/10.1080/15376516.2019.1701594
Reineke W, Schlömann M (2023) Heavy metals and other toxic inorganic ions. 331–348. https://doi.org/10.1007/978-3-662-66547-3_9
Kim JJ, Kim YS, Kumar V (2019) Heavy metal toxicity: An update of chelating therapeutic strategies. J Trace Elem Med Biol 54:226–231. https://doi.org/10.1016/j.jtemb.2019.05.003
Isangedighi IA, David GS (2019) Heavy metals contamination in fish: Effects on human health. J Aquat Sci Mar Biol 2
Giller KE, Witter E, McGrath SP (2009) Heavy metals and soil microbes. Soil Biol Biochem 41:2031–2037. https://doi.org/10.1016/J.SOILBIO.2009.04.026
Ramanujam J, Singh UP (2017) Copper indium gallium selenide based solar cells - A review. Energy Environ Sci 10:1306–1319. https://doi.org/10.1039/c7ee00826k
Kute AD, Gaikwad RP, Warkad IR, Gawande MB (2022) A review on the synthesis and applications of sustainable copper-based nanomaterials. Green Chem 24:3502–3573. https://doi.org/10.1039/D1GC04400A
Tuan WH, Lee SK (2014) Eutectic bonding of copper to ceramics for thermal dissipation applications – A review. J Eur Ceram Soc 34:4117–4130. https://doi.org/10.1016/J.JEURCERAMSOC.2014.07.011
Li X, Wang Y, Yin C, Yin Z (2020) Copper nanowires in recent electronic applications: Progress and perspectives. J Mater Chem C 8:849–872. https://doi.org/10.1039/C9TC04744A
Festa RA, Thiele DJ (2011) Copper: An essential metal in biology. Curr Biol 21. https://doi.org/10.1016/J.CUB.2011.09.040
Gunturu S, Dharmarajan TS (2020) Copper and zinc. Geriatr Gastroenterol 1–17. https://doi.org/10.1007/978-3-319-90761-1_25-1
Jomova K, Makova M, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, Rhodes CJ, Valko M (2022) Essential metals in health and disease. Chem Biol Interact 367 https://doi.org/10.1016/J.CBI.2022.110173
Uauy R, Olivares M, Gonzalez M (1998) Essentiality of copper in humans. Am J Clin Nutr 67:952S–959S. https://doi.org/10.1093/ajcn/67.5.952S
Royer A, Sharman T (2021) Copper Toxicity, StatPearls Publishing. http://www.ncbi.nlm.nih.gov/pubmed/32491388. Accessed 17 April 2021
Uriu-Adams JY, Keen CL (2005) Copper, oxidative stress, and human health. Mol Aspects Med 26:268–298. https://doi.org/10.1016/J.MAM.2005.07.015
Brewer GJ (2012) Copper toxicity in Alzheimer’s disease: Cognitive loss from ingestion of inorganic copper. J Trace Elem Med Biol 26:89–92. https://doi.org/10.1016/j.jtemb.2012.04.019
Barillo DJ, Marx DE (2014) Silver in medicine: A brief history BC 335 to present. Burns 40:S3–S8. https://doi.org/10.1016/J.BURNS.2014.09.009
Al-Saidi HM, Khan S (2022) A review on organic fluorimetric and colorimetric chemosensors for the detection of Ag(I) ions. Crit Rev Anal Chem. https://doi.org/10.1080/10408347.2022.2133561
Miyayama T, Arai Y, Hirano S (2016) Health effects of silver nanoparticles and silver ions. Biol Eff Fibrous Part Subst 137–147. https://doi.org/10.1007/978-4-431-55732-6_7
Hadrup N, Lam HR (2014) Oral toxicity of silver ions, silver nanoparticles and colloidal silver - A review. Regul Toxicol Pharmacol 68:1–7. https://doi.org/10.1016/j.yrtph.2013.11.002
Drake PL, Hazelwood KJ (2005) Exposure-related health effects of silver and silver compounds: A review. Ann Occup Hyg 49:575–585. https://doi.org/10.1093/ANNHYG/MEI019
Panyala NR, Peña-Méndez EM, Havel J (2008) Silver or silver nanoparticles: A hazardous threat to the environment and human health? J Appl Biomed 6:117–129. www.zsf.jcu.cz/jab. Accessed 18 May 2021
Qu H, Mudalige TK, Linder SW (2016) Capillary electrophoresis coupled with inductively coupled mass spectrometry as an alternative to cloud point extraction based methods for rapid quantification of silver ions and surface coated silver nanoparticles. J Chromatogr A 1429:348–353. https://doi.org/10.1016/J.CHROMA.2015.12.033
Tung NH, Chikae M, Ukita Y, Viet PH, Takamura Y (2012) Sensing technique of silver nanoparticles as labels for immunoassay using liquid electrode plasma atomic emission spectrometry. Anal Chem 84:1210–1213. https://doi.org/10.1021/AC202782B/SUPPL_FILE/AC202782B_SI_001.PDF
Mohammadi SZ, Afzali D, Taher MA, Baghelani YM (2009) Ligandless dispersive liquid–liquid microextraction for the separation of trace amounts of silver ions in water samples and flame atomic absorption spectrometry determination. Talanta 80:875–879. https://doi.org/10.1016/J.TALANTA.2009.08.009
Wang S, Forzani ES, Tao N (2007) Detection of heavy metal ions in water by high-resolution surface plasmon resonance spectroscopy combined with anodic stripping voltammetry. Anal Chem 79:4427–4432. https://doi.org/10.1021/AC0621773/SUPPL_FILE/AC0621773SI20070226_071019.PDF
Otero-Romaní J, Moreda-Piñeiro A, Bermejo-Barrera P, Martin-Esteban A (2009) Inductively coupled plasma–optical emission spectrometry/mass spectrometry for the determination of Cu, Ni, Pb and Zn in seawater after ionic imprinted polymer based solid phase extraction. Talanta 79:723–729. https://doi.org/10.1016/J.TALANTA.2009.04.066
Ghaedi M, Mortazavi K, Montazerozohori M, Shokrollahi A, Soylak M (2013) Flame atomic absorption spectrometric (FAAS) determination of copper, iron and zinc in food samples after solid-phase extraction on Schiff base-modified duolite XAD 761. Mater Sci Eng C 33:2338–2344. https://doi.org/10.1016/J.MSEC.2013.01.062
Sardans J, Montes F, Peñuelas J (2010) Determination of As, Cd, Cu, Hg and Pb in biological samples by modern electrothermal atomic absorption spectrometry, Spectrochim. Acta - Part B Spectrosc 65:97–112. https://doi.org/10.1016/J.SAB.2009.11.009
Yang GY, Li Z, Shi HL, Wang J (2005) Study on the determination of heavy-metal ions in tobacco and tobacco additives by microwave digestion and HPLC with PAD detection. J Anal Chem 605(60):480–485. https://doi.org/10.1007/S10809-005-0123-9
Stojanović Z, Koudelkova Z, Sedlackova E, Hynek D, Richtera L, Adam V (2018) Determination of chromium(VI) by anodic stripping voltammetry using a silver-plated glassy carbon electrode. Anal Methods 10:2917–2923. https://doi.org/10.1039/C8AY01047A
Alhamami MAM, Algethami JS, Khan S (2023) A review on thiazole based colorimetric and fluorimetric chemosensors for the detection of heavy metal ions. Crit Rev Anal Chem. https://doi.org/10.1080/10408347.2023.2197073
Algethami JS (2022) A review on recent progress in organic fluorimetric and colorimetric chemosensors for the detection of Cr 3+/6+ Ions. Crit Rev Anal Chem 1–21. https://doi.org/10.1080/10408347.2022.2082242
Khan S, Chen X, Almahri A, Allehyani ES, Alhumaydhi FA, Ibrahim MM, Ali S (2021) Recent developments in fluorescent and colorimetric chemosensors based on schiff bases for metallic cations detection: A review. J Environ Chem Eng 9. https://doi.org/10.1016/J.JECE.2021.106381
Berhanu AL, Gaurav I, Mohiuddin AK, Malik JS, Aulakh V, Kumar KH, Kim A (2019) Review of the applications of Schiff bases as optical chemical sensors. TrAC - Trends Anal Chem 116:74–91. https://doi.org/10.1016/j.trac.2019.04.025
Hussain Z, Yousif E, Ahmed A, Altaie A (2014) Synthesis and characterization of Schiff’s bases of sulfamethoxazole. Org Med Chem Lett 41(4):1–4. https://doi.org/10.1186/2191-2858-4-1
Kaya I, Solak E, Kamaci M (2021) Synthesis and multicolor, photophysical, thermal, and conductivity properties of poly(imine)s. J Taiwan Inst Chem Eng 123:328–337. https://doi.org/10.1016/J.JTICE.2021.05.010
Saydam S (2007) Synthesis and characterisation of the new thiazole schiff base 2-(2-hydroxy)naphthylideneamino-benzothiazole and its complexes with Co(II), Cu(II), AND Ni(II) ions. 32:437–447. https://doi.org/10.1081/SIM-120003787
Hassan AM, Said AO, Heakal BH, Younis A, Aboulthana WM, Mady MF (2022) Green synthesis, characterization, antimicrobial and anticancer screening of new metal complexes incorporating schiff base, ACS. Omega 7:32418–32431. https://doi.org/10.1021/ACSOMEGA.2C03911/ASSET/IMAGES/LARGE/AO2C03911_0009.JPEG
Al-Harbi S, Al-Harbi SA, Bashandy MS, Al-Saidi HM, Emara AAA, El-Gilil SMA (2016) Spectroscopic properties, anti-colon cancer, antimicrobial and molecular docking studies of silver(I), manganese(II), cobalt(II) and nickel(II) complexes for 2-amino-4-phenylthiazole derivative. Eur J Chem 7:421–430. https://doi.org/10.5155/eurjchem.7.4.421-430.1490
Mergu N, Gupta VK (2015) A novel colorimetric detection probe for copper(II) ions based on a Schiff base. Sens Actuators B Chem 210:408–417. https://doi.org/10.1016/J.SNB.2014.12.130
Zhu Wan-Yu, Liu Kai, Zhang Xuan (2023) A benzimidazole-derived fluorescent chemosensor for Cu( ii )-selective turn-off and Zn( ii )-selective ratiometric turn-on detection in aqueous solutions. Sens Diagnos 2:665–675. https://doi.org/10.1039/D3SD00020F
Wang L, Bing Q, Li J, Wang G (2018) A new “ON-OFF” fluorescent and colorimetric chemosensor based on 1,3,4-oxadiazole derivative for the detection of Cu2+ ions. J Photochem Photobiol A Chem 360:86–94. https://doi.org/10.1016/J.JPHOTOCHEM.2018.04.015
Affrose A, Parveen SDS, Kumar BS, Pitchumani K (2015) Selective sensing of silver ion using berberine, a naturally occurring plant alkaloid. Sens Actuators B Chem 206:170–175. https://doi.org/10.1016/J.SNB.2014.09.042
Bhuvanesh N, Suresh S, Kumar PR, Mothi EM, Kannan K, Kannan VR, Nandhakumar R (2018) Small molecule “turn on” fluorescent probe for silver ion and application to bioimaging. J Photochem Photobiol A Chem 360:6–12. https://doi.org/10.1016/J.JPHOTOCHEM.2018.04.027
Rout K, Manna AK, Sahu M, Mondal J, Singh SK, Patra GK (2019) Triazole-based novel bis Schiff base colorimetric and fluorescent turn-on dual chemosensor for Cu 2+ and Pb 2+: Application to living cell imaging and molecular logic gates. RSC Adv 9:25919–25931. https://doi.org/10.1039/C9RA03341F
Liu Y, Jiang B, Zhao L, Zhao L, Wang Q, Wang C, Xu B (2021) A dansyl-based fluorescent probe for sensing Cu2+ in aqueous solution. Spectrochim Acta Part A Mol Biomol Spectrosc 261. https://doi.org/10.1016/J.SAA.2021.120009
Wu YC, Jiang K, Luo SH, Cao L, Wu HQ, Wang ZY (2019) Novel dual-functional fluorescent sensors based on bis(5,6-dimethylbenzimidazole) derivatives for distinguishing of Ag+ and Fe3+ in semi-aqueous medium. Spectrochim Acta Part A Mol Biomol Spectrosc 206:632–641. https://doi.org/10.1016/J.SAA.2018.05.069
Zhang Y, Wang D, Sun C, Feng H, Zhao D, Bi Y (2017) A simple 2,6-diphenylpyridine-based fluorescence “turn-on” chemosensor for Ag+ with a high luminescence quantum yield. Dye Pigment 141:202–208. https://doi.org/10.1016/J.DYEPIG.2017.02.028
Karkosik A, Moro AJ (2022) An NIR emissive donor-π-acceptor dicyanomethylene-4h-pyran derivative as a fluorescent chemosensor system towards copper (II) detection. Chemosensors 10:343. https://doi.org/10.3390/CHEMOSENSORS10080343/S1
Lin Q, Yang Q, Sun B, Wei T, Zhang Y (2014) A novel highly selective “Turn-On” fluorescence sensor for silver ions based on Schiff base. Chin J Chem 32:1255–1258. https://doi.org/10.1002/cjoc.201400601
Yang JY, Gao WY, Wang JH, Dong ZM, Wang Y, Shuang SM (2023) A new NBD-based probe for specific colorimetric and turn-on fluorescence sensing of Cu2+ and bio-imaging applications. J Lumin 254. https://doi.org/10.1016/J.JLUMIN.2022.119549
Zhang S, Wu X, Niu Q, Guo Z, Li T, Liu H (2005) Highly selective and sensitive colorimetric and fluorescent chemosensor for rapid detection of Ag +, Cu 2+ and Hg 2+ based on a simple Schiff base. Springer 27:729–737. https://doi.org/10.1007/s10895-016-2005-y
Liu L, Liu X, Guo C, Fang M, Li C, Zhu W (2021) Carbazole-based dual-functional chemosensor: Colorimetric sensor for Co2+ and fluorescent sensor for Cu2+ and its application. J Chin Chem Soc 68:2368–2377. https://doi.org/10.1002/JCCS.202100343
Tümay SO (2021) A novel selective “Turn-On” fluorescent chemosensor based on thiophene appended cyclotriphosphazene Schiff base for detection of Ag+ ions. Chem Select 6:10561–10572. https://doi.org/10.1002/SLCT.202102052
Khan S, Muhammad M, Kamran AW, Al-Saidi HM, Alharthi SS, Algethami JS (2023) An ultrasensitive colorimetric and fluorescent “turn-on” chemosensor based on Schiff base for the detection of Cu2+ in the aqueous medium. Environ Monit Assess 195. https://doi.org/10.1007/S10661-023-11260-3
Chen C, Liu H, Zhang B, Wang Y, Cai K, Tan Y, Gao C, Liu H, Tan C, Jiang Y (2016) A simple benzimidazole quinoline-conjugate fluorescent chemosensor for highly selective detection of Ag+. Tetrahedron 72:3980–3985. https://doi.org/10.1016/J.TET.2016.05.020
Slassi S, Aarjane M, Amine A (2021) A novel imidazole-derived Schiff base as selective and sensitive colorimetric chemosensor for fluorescent detection of Cu2+ in methanol with mixed aqueous medium. Appl Organomet Chem 35. https://doi.org/10.1002/AOC.6408
Wang L, Zhang C, He H, Zhu H, Guo W, Zhou S, Wang S, Zhao JR, Zhang J (2020) Cellulose-based colorimetric sensor with N, S sites for Ag+ detection. Int J Biol Macromol 163:593–602. https://doi.org/10.1016/j.ijbiomac.2020.07.018
Dongare PR, Gore AH, Kolekar GB, Ajalkar BD (2020) A Phenazine based colorimetric and fluorescent chemosensor for sequential detection of Ag+ and I− in aqueous media. Luminescence 35:231–242. https://doi.org/10.1002/BIO.3718
Rahimi H, Hosseinzadeh R, Tajbakhsh M (2021) A new and efficient pyridine-2,6-dicarboxamide-based fluorescent and colorimetric chemosensor for sensitive and selective recognition of Pb2+ and Cu2+. J Photochem Photobiol A Chem 407. https://doi.org/10.1016/J.JPHOTOCHEM.2020.113049
Nagarajan R, Ryoo HI, Vanjare BD, Choi NG, Lee KH (2021) Novel phenylalanine derivative-based turn-off fluorescent chemosensor for selective Cu2+ detection in physiological pH. J Photochem Photobiol A Chem 418:113435. https://doi.org/10.1016/J.JPHOTOCHEM.2021.113435
Anand T, Sivaraman G, Anandh P, Chellappa D, Govindarajan S (2014) Colorimetric and turn-on fluorescence detection of Ag(I) ion. Tetrahedron Lett 55:671–675. https://doi.org/10.1016/J.TETLET.2013.11.104
Cui W, Wang L, Xiang G, Zhou L, An X, Cao D (2015) A colorimetric and fluorescence “turn-off” chemosensor for the detection of silver ion based on a conjugated polymer containing 2,3-di(pyridin-2-yl)quinoxaline. Sensors Actuators B Chem 207:281–290. https://doi.org/10.1016/J.SNB.2014.10.072
Mani KS, Rajamanikandan R, Murugesapandian B, Shankar R, Sivaraman G, Ilanchelian M, Rajendran SP (2019) Coumarin based hydrazone as an ICT-based fluorescence chemosensor for the detection of Cu2+ ions and the application in HeLa cells. Spectrochim Acta Part A Mol Biomol Spectrosc 214:170–176. https://doi.org/10.1016/J.SAA.2019.02.020
Tharmaraj V, Devi S, Pitchumani K (2012) An intramolecular charge transfer (ICT) based chemosensor for silver ion using 4-methoxy-N-((thiophen-2-yl)methyl)benzenamine. Analyst 137:5320–5324. https://doi.org/10.1039/C2AN35721F
Warrier SB, Kharkar PS (2018) A coumarin based chemosensor for selective determination of Cu (II) ions based on fluorescence quenching. J Lumin 199:407–415. https://doi.org/10.1016/J.JLUMIN.2018.03.073
Velmurugan K, Suresh S, Santhoshkumar S, Saranya M, Nandhakumar R (2016) A simple chalcone-based ratiometric chemosensor for silver ion. Luminescence 31:722–727. https://doi.org/10.1002/BIO.3016
Wang J, Niu Q, Wei T, Li T, Hu T, Chen J, Qin X, Yang Q, Yang L (2020) Novel phenothiazine-based fast-responsive colori/fluorimetric sensor for highly sensitive, selective and reversible detection of Cu2+ in real water samples and its application as an efficient solid-state sensor. Microchem J 157. https://doi.org/10.1016/J.MICROC.2020.104990
Bhuvanesh N, Suresh S, Prabhu J, Kannan K, Kannan VR, Nandhakumar R (2018) Ratiometric fluorescent chemosensor for silver ion and its bacterial cell imaging. Opt Mater (Amst) 82:123–129. https://doi.org/10.1016/J.OPTMAT.2018.05.053
Zhang Y, Li H, Pu S (2020) A colorimetric and fluorescent probe based on diarylethene for dual recognition of Cu2+ and CO32- and its application. J Photoch Photobiol A Chem 400. https://doi.org/10.1016/J.JPHOTOCHEM.2020.112721
Zhao H, Ding H, Kang H, Fan C, Liu G, Pu S (2019) A solvent-dependent chemosensor for fluorimetric detection of Hg 2+ and colorimetric detection of Cu 2+ based on a new diarylethene with a rhodamine B unit. RSC Adv 9:42155–42162. https://doi.org/10.1039/C9RA08557B
Li WT, Wu GY, Qu WJ, Li Q, Lou JC, Lin Q, Yao H, Zhang YM, Wei TB (2017) A colorimetric and reversible fluorescent chemosensor for Ag+ in aqueous solution and its application in IMPLICATION logic gate. Sens Actuators B Chem 239:671–678. https://doi.org/10.1016/J.SNB.2016.08.016
Bao Z, Qin C, Wang JJ, Sun J, Dai L, Chen G, Mei F (2018) A sensitive and selective probe for visual detection of Cu2+ based on 1, 8-naphthalimidederivative. Sens Actuators B Chem 265:234–241. https://doi.org/10.1016/J.SNB.2018.03.050
Acknowledgements
The authors are thankful to the Deanship of Scientific Research at Najran University for funding this work, under the Research Groups Funding program grant code (NU/RG/SERC/12/5).
Funding
This research was funded by the Deanship of Scientific Research at Najran University, grant code (NU/RG/SERC/12/5).
Author information
Authors and Affiliations
Contributions
Gasem Mohammad Abu-Taweel: Writing Original Draft-Equal, Conceptualization-Equal, Hamed M. Al-Saidi: Data Curation-Equal, Formal analysis-Equal, Mubark Alshareef: Visualization-Equal, Editing-Equal, Mohsen A. M. Alhamami: Data Curation-Equal, Conceptualization-Equal, Jari S. Algethami: Supervision-Equal, Editing-Equal, Salman S. Alharthi: Data Curation-Equal, Editing-Equal.
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with human participants or animals, clinical trial registration, or plant reproducibility performed by any authors.
Consent to Publish
The authors have approved the paper and agree with its publication.
Consent to Participate
No applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abu-Taweel, G.M., Al-Saidi, H.M., Alshareef, M. et al. Colorimetric Detection of Cu2+ and Ag+ Ions Using Multi-Responsive Schiff Base Chemosensor: A Versatile Approach for Environmental Monitoring. J Fluoresc (2023). https://doi.org/10.1007/s10895-023-03512-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-023-03512-9