Abstract
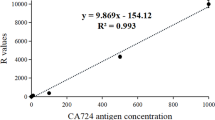
To establish a rapid and highly sensitive assay for tumor-associated trypsinogen-2 (TAT-2) based on the time-resolved fluorescence immunoassay (TRFIA) and evaluate its potential clinical value in patients with lung cancer. The double-antibody sandwich method was used in detecting TAT-2 antigen concentrations, and two types of TAT-2 antibodies (coating antibodies and Eu3+ labeled antibodies) were used. A TAT-2–TRFIA method was then established, evaluated, and used in detecting the serum TAT-2 levels of healthy subjects and patients with lung cancer. The linear range of the TAT-2–TRFIA method was 1.53–300 ng/mL, the intra-assay coefficient of variation (CV) were between 1.67% and 8.42%, and the inter-assay CV were between 4.29% and 11.44%. The recovery rates of TAT-2–TRFIA were between 99.17% and 107.06%. The cross-reactivities of trypsin and T-cell immunoglobulin mucin 3 were 0.02% and 0.82%, respectively. The serum TAT-2 levels of patients with lung cancer were higher than those of healthy subjects (P < 0.001). Combined with TAT-2, the sensitivity and specificity of CEA and CA-125 for lung cancer improved significantly.
Conclusion: We successfully established a highly sensitive TAT-2–TRFIA method, which was able to facilitate the timely diagnosis of lung cancer.




Similar content being viewed by others
Availability of Data and Material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Pham D, Bhandari S, Pinkston C, Oechsli M, Kloecker G (2020) Lung Cancer Screening Registry Reveals Low-dose CT Screening Remains Heavily Underutilized. Clin Lung Cancer 21(3):e206–e211
Ruchalski K, Gutierrez A, Genshaft S, Abtin F, Suh R (2016) The evidence for low-dose CT screening of lung cancer. Clin Imaging 40(2):288–295
Shen H (2018) Low-dose CT for lung cancer screening: opportunities and challenges. Front Med 12(1):116–121
Collins LG, Haines C, Perkel R, Enck RE (2007) Lung cancer: diagnosis and management. Am Fam Physician 75(1):56–63
Maclay JD, Farley JM, McCowan C, Tweed C, Milroy R (2017) Obtaining tissue diagnosis in lung cancer patients with poor performance status and its influence on treatment and survival. Respir Med 124:30–35
Nanavaty P, Alvarez MS, Alberts WM (2014) Lung cancer screening: advantages, controversies, and applications. Cancer Control 21(1):9–14
Jiang JC, Zhang Y (2020) Serological antibody testing in the COVID-19 pandemic: their molecular basis and applications. Biochem Soc Trans 48(6):2851–2863
Lau A, Chen S, Sleiman S, Sorrell T (2009) Current status and future perspectives on molecular and serological methods in diagnostic mycology. Future Microbiol 4(9):1185–1222
Li X, Asmitananda T, Gao L, Gai D, Song Z, Zhang Y, Ren H, Yang T, Chen T, Chen M (2012) Biomarkers in the lung cancer diagnosis: a clinical perspective. Neoplasma 59(5):500–507
Wang R, Wang G, Zhang N, Li X, Liu Y (2013) Clinical evaluation and cost-effectiveness analysis of serum tumor markers in lung cancer. Biomed Res Int 2013:195692
Burt RW, Ratcliffe JG, Stack BH, Cuthbert J, Kennedy RS, Corker CS, Franchimont P, Spilg WG, Stimson WH (1978) Serum biochemical markers in lung cancer. Br J Cancer 37(5):714–717
Isaksson S, Jönsson P, Monsef N, Brunnström H, Bendahl PO, Jönsson M, Staaf J, Planck M (2017) CA 19–9 and CA 125 as potential predictors of disease recurrence in resectable lung adenocarcinoma. PLoS ONE 12(10):e0186284
Grunnet M, Sorensen JB (2012) Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer 76(2):138–143
Itkonen O (2010) Human trypsinogens in the pancreas and in cancer. Scand J Clin Lab Invest 70(2):136–143
Kawano N, Osawa H, Ito T, Nagashima Y, Hirahara F, Inayama Y, Nakatani Y, Kimura S, Kitajima H, Koshikawa N et al (1997) Expression of gelatinase A, tissue inhibitor of metalloproteinases-2, matrilysin, and trypsin(ogen) in lung neoplasms: an immunohistochemical study. Hum Pathol 28(5):613–622
Kohn EC, Liotta LA (1995) Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res 55(9):1856–1862
Koivunen E, Ristimäki A, Itkonen O, Osman S, Vuento M, Stenman UH (1991) Tumor-associated trypsin participates in cancer cell-mediated degradation of extracellular matrix. Cancer Res 51(8):2107–2112
Sorsa T, Salo T, Koivunen E, Tyynelä J, Konttinen YT, Bergmann U, Tuuttila A, Niemi E, Teronen O, Heikkilä P et al (1997) Activation of type IV procollagenases by human tumor-associated trypsin-2. J Biol Chem 272(34):21067–21074
Yang X, Ye Y, Wang T, Li M, Yu L, Xia M, Qian J, Hu Z (2019) Eu(3+) /Sm(3+) dual-label time-resolved fluoroimmunoassay for measurement of hepatitis C virus antibodies. J Clin Lab Anal 33(2):e22659
Zhang Q, Huang B, Liu X, Liu B, Zhang Y, Zhang Z, Hua J, Fan Y, Hu L, Meng M et al (2017) Ultrasensitive quantitation of anti-phospholipase A2 receptor antibody as a diagnostic and prognostic indicator of idiopathic membranous nephropathy. Sci Rep 7(1):12049
Huang B, Xiao H, Zhang X, Zhu L, Liu H, Jin J (2006) Ultrasensitive detection of pepsinogen I and pepsinogen II by a time-resolved fluoroimmunoassay and its preliminary clinical applications. Anal Chim Acta 571(1):74–78
Koivunen E, Huhtala ML, Stenman UH (1989) Human ovarian tumor-associated trypsin. Its purification and characterization from mucinous cyst fluid and identification as an activator of pro-urokinase. J Biol Chem 264(24):14095–14099
Koivunen E, Saksela O, Itkonen O, Osman S, Huhtala ML, Stenman UH (1991) Human colon carcinoma, fibrosarcoma and leukemia cell lines produce tumor-associated trypsinogen. Int J Cancer 47(4):592–596
Lukkonen A, Sorsa T, Salo T, Tervahartiala T, Koivunen E, Golub L, Simon S, Stenman UH (2000) Down-regulation of trypsinogen-2 expression by chemically modified tetracyclines: association with reduced cancer cell migration. Int J Cancer 86(4):577–581
Stenman M, Paju A, Hanemaaijer R, Tervahartiala T, Leminen A, Stenman UH, Konttinen YT, Sorsa T (2003) Collagenases (MMP-1, -8 and -13) and trypsinogen-2 in fluid from benign and malignant ovarian cysts. Tumour Biol 24(1):9–12
Kitamura H, Oosawa Y, Kawano N, Kameda Y, Hayashi H, Nakatani Y, Udaka N, Ito T, Miyazaki K (1999) Basement membrane patterns, gelatinase A and tissue inhibitor of metalloproteinase-2 expressions, and stromal fibrosis during the development of peripheral lung adenocarcinoma. Hum Pathol 30(3):331–338
Kimland M, Russick C, Marks WH, Borgström A (1989) Immunoreactive anionic and cationic trypsin in human serum. Clin Chim Acta 184(1):31–46
Huang B, Yang X, Zhang W, Wu J, Liu P, Hu Z, Wang T (2020) Receptor antibody time-resolved A2 phospholipase bead immunochromatography and its application in idiopathic membranous nephropathy. J Clin Lab Anal 34(12):e23508
Chen M, Wang L, Wang Y, Zhou X, Liu X, Chen H, Huang B, Hu Z (2020) Soluble Tim3 detection by time-resolved fluorescence immunoassay and its application in membranous nephropathy. J Clin Lab Anal 34(6):e23248
Funding
Funding was provided by the Social Development Fund of Zhejiang Province (No. LGF20H200008), the Key Research and Development Program of Zhejiang Province (No. 2020C03066), “Two Hundreds” Young and Middle-aged Medical Key Talent Project of Wuxi (No. BJ2020027), the Opening Project of Zhejiang Provincial Preponderant and Characteristic Subject of Key University (Traditional Chinese Pharmacology), Key Research and Development Project of Hangzhou (No.202004A23).
Author information
Authors and Affiliations
Contributions
Xindong Chen: Conceptualization, Methodology, Writing-Original Draft. Jianfeng Hong, Han Zhao: Software, Formal analysis. Zhongyi Xiang: Data Curation, Visualization. Yuan Qin, Xiumei Zhou, Yigang Wang, Liping Zheng, Biao Huang: Reviewing. Biao Huang, Pengguo Xia provided the funds. Biao Huang, Yingwei Zhu, Hongming Fang: Project administration and Supervision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
The overall design of this research was approved by Zhejiang Xiaoshan Hospital, and this research was approved by the ethics committee of Zhejiang Xiaoshan Hospital. Ethic number is 2020–011.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Patients signed informed consent regarding publishing their data and photographs.
Conflicts of Interest
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, X., Hong, J., Zhao, H. et al. Establishment and Clinical Application of a Highly Sensitive Time-Resolved Fluorescence Immunoassay for Tumor-Associated Trypsinogen-2. J Fluoresc 32, 1501–1507 (2022). https://doi.org/10.1007/s10895-022-02950-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-022-02950-1