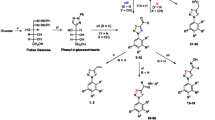
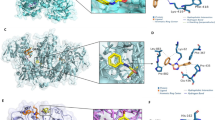
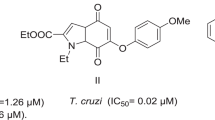
Abstract
Current treatment of Chagas disease (CD) is based on two substances, nifurtimox (NT) and benzonidazole (BZ), both considered unsatisfactory mainly due to their low activities and high toxicity profile. One of the main challenges faced in CD management concerns the identification of new drugs active in the acute and chronic phases and with good pharmacokinetic profiles. In this work, we studied the bioactivity of twenty 2-(1H-pyrazol-1-yl)-1,3,4-thiadiazole derivatives against Trypanosoma cruzi epimastigotes and trypomastigotes. We identified seven derivatives with promising activity against epimastigote forms with IC50 values ranging from 6 µM to 44 µM. Most of the compounds showed no significant toxicity against murine macrophages. Our initial investigation on the mechanism of action indicates that this series of compounds may exert their anti-parasitic effect, inducing cell membrane damage. The results in trypomastigotes showed that one derivative, PDAN 78, satisfactorily inhibited metabolic alteration at all concentrations. Moreover, we used molecular modeling to understand how tridimensional and structural aspects might influence the observed bioactivities. Finally, we also used in silico approaches to assess the potential pharmacokinetic and toxicological properties of the most active compounds. Our initial results indicate that this molecular scaffold might be a valuable prototype for novel and safe trypanocidal compounds.








Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the article and its supplementary information files.
References
Anthwal T, Paliwal S, Nain S (2022) Diverse biological activities of 1,3,4-Thiadiazole scaffold. Chemistry (Switzerland) 4. https://doi.org/10.3390/chemistry4040107
Banerjee P, Eckert AO, Schrey AK, Preissner R (2018) ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res 46. https://doi.org/10.1093/nar/gky318
Bonney KM, Luthringer DJ, Kim SA, Garg NJ, Engman DM (2019) Pathology and pathogenesis of Chagas heart disease. Ann Rev Pathol: Mech Dis 14. https://doi.org/10.1146/annurev-pathol-020117-043711
Camargo J do NA, Pianoski KE, dos Santos MG, Lazarin-Bidóia D, Volpato H, Moura S, Nakamura CV, Rosa FA (2020) Antiparasitic behavior of Trifluoromethylated Pyrazole 2-Amino-1,3,4-thiadiazole hybrids and their analogues: synthesis and structure-activity relationship. Front Pharmacol 11. https://doi.org/10.3389/fphar.2020.591570
Coura JR, Borges-Pereira J (2011) Chronic phase of Chagas disease: Why should it be treated? A comprehensive review. Mem Inst Oswaldo Cruz 106. https://doi.org/10.1590/S0074-02762011000600001
Crespillo-Andújar C, Comeche B, Hamer DH, Arevalo-Rodriguez I, Alvarez-Díaz N, Zamora J, Pérez-Molina JA (2022) Use of benznidazole to treat chronic Chagas disease: An updated systematic review with a meta-analysis. PLoS Negl Trop Dis 16:e0010386. https://doi.org/10.1371/journal.pntd.0010386
Dawood KM, Farghaly TA (2017) Thiadiazole inhibitors: a patent review. Expert Opin Ther Pat 27. https://doi.org/10.1080/13543776.2017.1272575
de Luna Martins D, Borges AA, do A e Silva NA, Faria JV, Hoelz LVB, de Souza HVCM, Bello ML, Boechat N, Ferreira VF, Faria RX (2020) P2X7 receptor inhibition by 2-amino-3-aryl-1,4-naphthoquinones. Bioorg Chem 104. https://doi.org/10.1016/j.bioorg.2020.104278
de Souza W (2009) Structural organization of Trypanosoma cruzi. Mem Inst Oswaldo Cruz 104:89–100. https://doi.org/10.1590/s0074-02762009000900014
Dong J, Wang NN, Yao ZJ, Zhang L, Cheng Y, Ouyang D, Lu AP, Cao DS (2018) Admetlab: A platform for systematic ADMET evaluation based on a comprehensively collected ADMET database. J Cheminform 10. https://doi.org/10.1186/s13321-018-0283-x
Dubin M, Goijman SG, Stoppani AOM (1984) Effect of nitroheterocyclic drugs on lipid peroxidation and glutathione content in rat liver extracts. Biochem Pharmacol 33. https://doi.org/10.1016/0006-2952(84)90114-X
Ebenezer O, Shapi M, Tuszynski JA (2022) A review of the recent development in the synthesis and biological evaluations of Pyrazole derivatives. Biomedicines 10. https://doi.org/10.3390/biomedicines10051124
Echeverria LE, Morillo CA (2019) American trypanosomiasis (Chagas Disease). Infect Dis Clin North Am 33:119–134
Freitas RHCN, Barbosa JMC, Bernardino P, Sueth-Santiago V, Wardell SMSV, Wardell JL, Decoté-Ricardo D, Melo TG, da Silva EF, Salomão K, Fraga CAM (2020) Synthesis and trypanocidal activity of novel pyridinyl-1,3,4-thiadiazole derivatives. Biomed Pharmacother 127. https://doi.org/10.1016/j.biopha.2020.110162
Gómez-Ochoa SA, Rojas LZ, Echeverría LE, Muka T, Franco OH (2022) Global, regional, and national trends of Chagas disease from 1990 to 2019: comprehensive analysis of the global burden of disease study. Glob Heart 17:59. https://doi.org/10.5334/gh.1150
Gonzaga DT, Oliveira FH, Salles JP, Bello ML, Rodrigues CR, Castro HC, de Souza H, Reis C, Leme R, Mafra J, Pinheiro L, Hoelz L, Boechat N, Faria RX (2019) Synthesis, biological evaluation and molecular modeling studies of new thiadiazole derivatives as potent P2X7 receptor inhibitors. Front Chem 7. https://doi.org/10.3389/fchem.2019.00261
Halgren TA (1996) Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J Comput Chem 17:490–519. https://doi.org/10.1002/(SICI)1096-987X(199604)17:5/6%3c490::AID-JCC1%3e3.0.CO;2-P
Hanwell MD, Curtis DE, Lonie DC, Vandermeerschd T, Zurek E, Hutchison GR (2012) Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J Cheminform 4. https://doi.org/10.1186/1758-2946-4-17
Hu J, Liu Z, Yu DJ, Zhang Y (2018) LS-align: An atom-level, flexible ligand structural alignment algorithm for high-throughput virtual screening. Bioinformatics. https://doi.org/10.1093/bioinformatics/bty081
Jain AK, Sharma S, Vaidya A, Ravichandran V, Agrawal RK (2013) 1,3,4-thiadiazole and its derivatives: a review on recent progress in biological activities. Chem Biol Drug Des 81. https://doi.org/10.1111/cbdd.12125
Li G, Cheng Y, Han C, Song C, Huang N, Du Y (2022) Pyrazole-containing pharmaceuticals: target, pharmacological activity, and their SAR studies. RSC Med Chem 13. https://doi.org/10.1039/d2md00206j
Martín-Escolano J, Marín C, Rosales MJ, Tsaousis AD, Medina-Carmona E, Martín-Escolano R (2022) An updated view of the Trypanosoma cruzi life cycle: intervention points for an effective treatment. ACS Infect Dis 8:1107–1115. https://doi.org/10.1021/acsinfecdis.2c00123
Naim MJ, Alam O, Nawaz F, Alam MJ, Alam P (2016) Current status of pyrazole and its biological activities. J Pharm Bioallied Sci 8. https://doi.org/10.4103/0975-7406.171694
Pacheco PAF, Faria JV, Silva AC, von Ranke NL, Silva RC, Rodrigues CR, da Rocha DR, Faria RX (2023) In silico and pharmacological study of N, S-acetal juglone derivatives as inhibitors of the P2X7 receptor-promoted in vitro and in vivo inflammatory response. Biomed Pharmacother 162:114608. https://doi.org/10.1016/j.biopha.2023.114608
Pan American Health Organization/World Health Organization (2023) Chagas disease. https://www.paho.org/en/topics/chagas-disease. Accessed 31 Mar 2023
Rassi A, Rassi A, Marcondes de Rezende J (2012) American trypanosomiasis (Chagas Disease). Infect Dis Clin North Am 26:275–291. https://doi.org/10.1016/j.idc.2012.03.002
Stewart JJP (2007) Optimization of parameters for semiempirical methods V: modification of NDDO approximations and application to 70 elements. J Mol Model 13:1173–1213. https://doi.org/10.1007/s00894-007-0233-4
Stewart JJP (2016) Stewart computational chemistry, Mopac2016. http://openmopac.net. Accessed 31 Mar 2023
Tyler KM, Engman DM (2001) The life cycle of Trypanosoma cruzi revisited. Int J Parasitol: 472–481. https://doi.org/10.1016/S0020-7519(01)00153-9
Zuma AA, de Souza W (2021) Chagas disease chemotherapy: what do we know so far? Curr Pharm Des 27:3963–3995. https://doi.org/10.2174/1381612827666210216152654
Funding
The fellowships granted by CNPq (316568/2021–0 and 163130/2013–2), CAPES (Financial Code 001) and FAPERJ (E-26/203.246/2017, E-26/200.982/2021, E-26/010.000984/2019, E-26/210.002/2020, and E-26/201.369/2021) are gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
These authors did the biological experimets—AFMF, CSFP, RMSG, GPT, DHJ, RXF
These authors did the substance sinthesis—DTGG, NB
This author did the in-silico assay—MLB, NLVR
These authors wrote the paper—AFMF, CSFP, GPT, RMSG, PAFP, GPT, DHJ, RXF
These authors revised the experiments- PAFP, KC, NB, RXF
These authors revised the manuscript- GPT, PAFP, KC, NB, RXF
These authors revised plainned the experiments—KC, NB, RXF
These authors revised received the funds—NB, RXF
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Natalia Lidmar von Ranke performed the in silico analyzes of this work and contributed as lead author.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Faria, A.F.M., de Souza Ferreira Pereira, C., Teixeira, G.P. et al. In vitro evaluation of 2-(1H-pyrazol-1-yl)-1,3,4-thiadiazole derivatives against replicative and infective stages of Trypanosoma cruzi. J Bioenerg Biomembr 55, 409–421 (2023). https://doi.org/10.1007/s10863-023-09982-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-023-09982-7