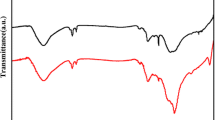
Abstract
A well-defined controlled selective edge functionalization of graphene oxide (GO) is of high interest because it allows tuning the chemical and physical properties of graphene oxide with minimal damage to the carbon at the basal plane. The present work reports a rapid one-step synthesis of edge fluorinated graphene oxide (FGO) from GO in an aqueous medium. A selective fluorination of the tertiary carbon at the edge of GO was achieved by chemoselective substitution of the carboxylic acid with fluorine in one hour following a decarboxylative fluorination technique using 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) (SELECTFLUOR) and silver ion catalyst. The structure and composition of FGO were characterized by multiple analytical techniques, such as TEM, SEM, XRD, EDS, FTIR, XPS, Raman spectroscopy, etc. As observed in XPS and NMR analysis, the decarboxylative fluorination of GO resulted in the formation of covalent C–F bonds at the edge. The absence of the peak associated with the C–F group on the basal plane in 19F NMR clearly indicates the fluorination at the edge of GO. Most importantly, similar linewidth and spectral patterns in proton-decoupled 19F{1H} and proton-coupled 19F NMR spectra of FGO suggest that the fluorine atoms are bonded to the tertiary carbon atom. The selective functionalization of the tertiary carbon at the edges of GO achieved here, is unprecedented. The fluorine group at the edge of GO can act as a new reaction center for subsequent chemical modification. This simple edge-controlled fabrication method described here provides a facile pathway to fabricate multifunctional GO and expand their potential applications.
Graphical abstract





Similar content being viewed by others
Data availability
The data that support the findings of this study are available. All data generated or analysed during this study are included in this published article.
References
Bhattacharjya D, Jeon I-Y, Park H-Y et al (2015) Graphene nanoplatelets with selectively functionalized edges as electrode material for electrochemical energy storage. Langmuir 31:5676–5683. https://doi.org/10.1021/acs.langmuir.5b00195
Jeon I-Y, Choi H-J, Ju MJ et al (2013) Direct nitrogen fixation at the edges of graphene nanoplatelets as efficient electrocatalysts for energy conversion. Sci Rep 3:2260. https://doi.org/10.1038/srep02260
Jeon I-Y, Shin Y-R, Sohn G-J et al (2012) Edge-carboxylated graphene nanosheets via ball milling. Proc Natl Acad Sci 109:5588–5593. https://doi.org/10.1073/pnas.1116897109
Jeon I-Y, Choi H-J, Jung S-M et al (2013) Large-scale production of edge-selectively functionalized graphene nanoplatelets via ball milling and their use as metal-free electrocatalysts for oxygen reduction reaction. J Am Chem Soc 135:1386–1393. https://doi.org/10.1021/ja3091643
Li M, Zhou S, Ren S et al (2022) Precise edge functionalization and tailoring of graphene via solvent-controlled reactions. Carbon 197:519–525. https://doi.org/10.1016/j.carbon.2022.06.072
Sun Z, Kohama S, Zhang Z et al (2010) Soluble graphene through edge-selective functionalization. Nano Res 3:117–125. https://doi.org/10.1007/s12274-010-1016-2
Tan Y-Z, Yang B, Parvez K et al (2013) Atomically precise edge chlorination of nanographenes and its application in graphene nanoribbons. Nat Commun 4:2646–2652. https://doi.org/10.1038/ncomms3646
Chua CK, Pumera M (2012) Friedel—crafts acylation on graphene. Chem Asian J 7:1009–1012. https://doi.org/10.1002/asia.201200096
Agarwal N, Bhattacharyya R, Tripathi NK et al (2017) Derivatization and interlaminar debonding of graphite–iron nanoparticle hybrid interfaces using fenton chemistry. Phys Chem Chem Phys 19:16329–16336. https://doi.org/10.1039/C7CP00357A
Zhou X, Zhang Y, Wang C et al (2012) Photo-fenton reaction of graphene oxide: a new strategy to prepare graphene quantum dots for DNA cleavage. ACS Nano 6:6592–6599. https://doi.org/10.1021/nn301629v
Margani F, Magrograssi M, Piccini M et al (2022) Facile edge functionalization of graphene layers with a biosourced 2-pyrone. ACS Sustain Chem Eng 10:4082–4093. https://doi.org/10.1021/acssuschemeng.1c06182
Lerf A, He H, Forster M, Klinowski J (1998) Structure of graphite oxide revisited. J Phys Chem B 102:4477–4482. https://doi.org/10.1021/jp9731821
Zhao F-G, Zhao G, Liu X-H et al (2014) Fluorinated graphene: facile solution preparation and tailorable properties by fluorine-content tuning. J Mater Chem A 2:8782–8789. https://doi.org/10.1039/C4TA00847B
Nair RR, Ren W, Jalil R et al (2010) Fluorographene: a two-dimensional counterpart of teflon. Small 6:2877–2884. https://doi.org/10.1002/smll.201001555
Hong X, Cheng S-H, Herding C, Zhu J (2011) Colossal negative magnetoresistance in dilute fluorinated graphene. Phys Rev B 83:085410. https://doi.org/10.1103/PhysRevB.83.085410
Cheng S-H, Zou K, Okino F et al (2010) Reversible fluorination of graphene: evidence of a two-dimensional wide bandgap semiconductor. Phys Rev B 81:205435. https://doi.org/10.1103/PhysRevB.81.205435
Yang Y, Lu G, Li Y et al (2013) One-step preparation of fluorographene: a highly efficient, low-cost, and large-scale approach of exfoliating fluorographite. ACS Appl Mater Interfaces 5:13478–13483. https://doi.org/10.1021/am405046u
Liu Y, Li J, Chen X, Luo J (2019) Fluorinated graphene: a promising macroscale solid lubricant under various environments. ACS Appl Mater Interfaces 11:40470–40480. https://doi.org/10.1021/acsami.9b13060
Arshad MU, Dutta D, Sin YY et al (2022) Multi-functionalized fluorinated graphene composite coating for achieving durable electronics: ultralow corrosion rate and high electrical insulating passivation. Carbon 195:141–153. https://doi.org/10.1016/j.carbon.2022.04.004
Jeong E, Jung S, Shin H-S (2023) Fluorine-functionalized reduced graphene oxide-TiO2 nanocomposites: a new application approach for efficient photocatalytic disinfection and algicidal effect. Environ Pollut 319:120974. https://doi.org/10.1016/j.envpol.2022.120974
Kumar S, Arumugham H, Roy D, Kannaiyan D (2022) Synthesis and characterization of fluorine functionalized graphene oxide dispersed quinoline-based polyimide composites having low-k and UV shielding properties. Polym Adv Technol 33:427–439. https://doi.org/10.1002/pat.5527
Sun C, Feng Y, Li Y et al (2014) Solvothermally exfoliated fluorographene for high-performance lithium primary batteries. Nanoscale 6:2634–2641. https://doi.org/10.1039/C3NR04609E
Ho K-I, Huang C-H, Liao J-H et al (2014) Fluorinated graphene as high performance dielectric materials and the applications for graphene nanoelectronics. Sci Rep 4:5893. https://doi.org/10.1038/srep05893
Jiang Y, Wang H, Baek J et al (2022) Perfluoroalkyl-functionalized graphene oxide as a multifunctional additive for promoting the energetic performance of aluminum. ACS Nano 16:14658–14665. https://doi.org/10.1021/acsnano.2c05271
Park KT, Choi J, Sung SJ et al (2021) Surface energy modification of graphene oxide film by silanization co-functionalized with fluorine to maximize the moisture barrier property. Synth Met 277:116770. https://doi.org/10.1016/j.synthmet.2021.116770
Wang Y, Lee WC, Manga KK et al (2012) Fluorinated graphene for promoting neuro-induction of stem cells. Adv Mater 24:4285–4290. https://doi.org/10.1002/adma.201200846
Romero-Aburto R, TharangattuN N, Nagaoka Y et al (2013) Fluorinated graphene oxide; a new multimodal material for biological applications. Adv Mater 25:5632–5637. https://doi.org/10.1002/adma201301804
Jankovský O, Šimek P, Sedmidubský D et al (2013) Water-soluble highly fluorinated graphite oxide. RSC Adv 4:1378–1387. https://doi.org/10.1039/C3RA45183F
Jeon K-J, Lee Z, Pollak E et al (2011) Fluorographene: a wide bandgap semiconductor with ultraviolet luminescence. ACS Nano 5:1042–1046. https://doi.org/10.1021/nn1025274
Min C, He Z, Song H et al (2019) Fluorinated graphene oxide nanosheet: a highly efficient water-based lubricated additive. Tribol Int 140:105867–105875. https://doi.org/10.1016/j.triboint.2019.105867
Lee WH, Suk JW, Chou H et al (2012) Selective-area fluorination of graphene with fluoropolymer and laser irradiation. Nano Lett 12:2374–2378. https://doi.org/10.1021/nl300346j
Chen M, Chen M, Qiu C et al (2013) Fluorination of edges and central areas of monolayer graphene by SF6 and CHF3 plasma treatments. J Nanosci Nanotechnol 13:1331–1334. https://doi.org/10.1166/jnn.2013.5996
Johns JE, Hersam MC (2013) Atomic covalent functionalization of graphene. Acc Chem Res 46:77–86. https://doi.org/10.1021/ar300143e
Zbořil R, Karlický F, Bourlinos AB et al (2010) Graphene fluoride: a stable stoichiometric graphene derivative and its chemical conversion to graphene. Small 6:2885–2891. https://doi.org/10.1002/smll.201001401
Yang R, Zhang L, Wang Y et al (2010) An anisotropic etching effect in the graphene basal plane. Adv Mater 22:4014–4019. https://doi.org/10.1002/adma.201000618
Hod O, Barone V, Peralta JE, Scuseria GE (2007) Enhanced half-metallicity in edge-oxidized zigzag graphene nanoribbons. Nano Lett 7:2295–2299. https://doi.org/10.1021/nl0708922
Yan Q, Huang B, Yu J et al (2007) Intrinsic current−voltage characteristics of graphene nanoribbon transistors and effect of edge doping. Nano Lett 7:1469–1473. https://doi.org/10.1021/nl070133j
Compton OC, Nguyen ST (2010) Graphene oxide, highly reduced graphene oxide, and graphene: versatile building blocks for carbon-based materials. Small 6:711–723. https://doi.org/10.1002/smll.200901934
Eda G, Chhowalla M (2010) Chemically derived graphene oxide: towards large-area thin-film electronics and optoelectronics. Adv Mater 22:2392–2415. https://doi.org/10.1002/adma.200903689
Shellard PM, Srisubin T, Hartmann M et al (2020) A versatile route to edge-specific modifications to pristine graphene by electrophilic aromatic substitution. J Mater Sci 55:10284–10302. https://doi.org/10.1007/s10853-020-04662-y
Zhang J, Ren Y, Xu T et al (2015) Liquid crystal graphene oxide with different layers: fabrication, characterization and applications. RSC Adv 5:94809–94813. https://doi.org/10.1039/C5RA16539C
William S, Hummers J, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339–1339. https://doi.org/10.1021/ja01539a017
Yin F, Wang Z, Li Z, Li C (2012) Silver-catalyzed decarboxylative fluorination of aliphatic carboxylic acids in aqueous solution. J Am Chem Soc 134:10401–10404. https://doi.org/10.1021/ja3048255
Gong P, Wang Z, Li Z et al (2013) Photochemical synthesis of fluorinated graphene via a simultaneous fluorination and reduction route. RSC Adv 3:6327–6330. https://doi.org/10.1039/C3RA22029J
Hussain S, Dam S (2021) Synthesis of vertically stacked, highly oriented WS2 thin films by electron beam evaporation. Thin Solid Films 734:138851–138858. https://doi.org/10.1016/j.tsf.2021.138851
Mazánek V, Jankovský O, Luxa J et al (2015) Tuning of fluorine content in graphene: towards large-scale production of stoichiometric fluorographene. Nanoscale 7:13646–13655. https://doi.org/10.1039/C5NR03243A
Xing R, Li Y, Yu H (2015) Preparation of fluoro-functionalized graphene oxide via the hunsdiecker reaction. Chem Commun 52:390–393. https://doi.org/10.1039/C5CC08252H
Park M-S, Lee Y-S (2016) Functionalization of graphene oxide by fluorination and its characteristics. J Fluor Chem 182:91–97. https://doi.org/10.1016/j.jfluchem.2015.12.011
Wallace PR (1947) The band theory of graphite. Phys Rev 71:622–634. https://doi.org/10.1103/PhysRev.71.622
Fan X, Peng W, Li Y et al (2008) Deoxygenation of exfoliated graphite oxide under alkaline conditions: a green route to graphene preparation. Adv Mater 20:4490–4493. https://doi.org/10.1002/adma.200801306
Sohail M, Saleem M, Ullah S et al (2017) Modified and improved Hummer’s synthesis of graphene oxide for capacitors applications. Mod Electron Mater 3:110–116. https://doi.org/10.1016/j.moem.2017.07.002
Asanov IP, Bulusheva LG, Dubois M et al (2013) Graphene nanochains and nanoislands in the layers of room-temperature fluorinated graphite. Carbon 59:518–529. https://doi.org/10.1016/j.carbon.2013.03.048
Ferrari AC, Meyer JC, Scardaci V et al (2006) Raman spectrum of graphene and graphene layers. Phys Rev Lett 97:187401. https://doi.org/10.1103/PhysRevLett.97.187401
Maiti S, Kundu S, Ghosh D et al (2016) Synthesis and spectral measurements of sulphonated graphene: some anomalous observations. Phys Chem Chem Phys 18:6701–6705. https://doi.org/10.1039/C5CP05799J
Kudin KN, Ozbas B, Schniepp HC et al (2008) Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett 8:36–41. https://doi.org/10.1021/nl071822y
Roy D, Kanojia S, Mukhopadhyay K, Eswara Prasad N (2021) Analysis of carbon-based nanomaterials using Raman spectroscopy: principles and case studies. Bull Mater Sci 44:31. https://doi.org/10.1007/s12034-020-02327-9
Sato Y, Itoh K, Hagiwara R et al (2004) On the so-called “semi-ionic” C–F bond character in fluorine–GIC. Carbon 42:3243–3249. https://doi.org/10.1016/j.carbon.2004.08.012
Patel NR, Flowers RA (2015) Mechanistic study of silver-catalyzed decarboxylative fluorination. J Org Chem 80:5834–5841. https://doi.org/10.1021/acs.joc.5b00826
Chronopoulos DD, Bakandritsos A, Lazar P et al (2017) High-yield alkylation and arylation of graphene via grignard reaction with fluorographene. Chem Mater 29:926–930. https://doi.org/10.1021/acs.chemmater.6b05040
Bakandritsos A, Pykal M, Błoński P et al (2017) Cyanographene and graphene acid: emerging derivatives enabling high-yield and selective functionalization of graphene. ACS Nano 11:2982–2991. https://doi.org/10.1021/acsnano.6b08449
Acknowledgements
TKD acknowledges CSIR, India for his doctoral fellowship and SK is grateful to DST India for DST-INSPIRE Fellowship.
Author information
Authors and Affiliations
Contributions
TKD did methodology development, investigation, and formal analysis; SK performed investigation; PG done investigation and formal analysis; SB provided investigation and formal analysis; UD provided methodology development and formal analysis; SH did methodology development, investigation, and formal analysis; SP performed methodology development, formal analysis, writing—review & editing; NK done methodology development; AS updated supervision, writing—review & editing; GP performed conceptualization, project administration, supervision, writing-review & editing.
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Ethical approval
Not applicable.
Supplementary information
Supplementary information is available: synthesis of GO from graphite; Zeta Potential graph; SEM EDS spectra; FTIR spectra; Raman spectra.
Additional information
Handling Editor: Christopher Blanford.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Das, T.K., Karmakar, S., Garg, P. et al. Fluorination of the tertiary carbon at the edge of graphene oxide. J Mater Sci 58, 9409–9419 (2023). https://doi.org/10.1007/s10853-023-08582-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-08582-5