Abstract
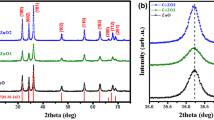
Biodegradable potato starch-assisted nanoferrite and ferrite–semiconductor nanocomposites have been synthesized by the co-precipitation method for the degradation of MB dye under visible light irradiation. Ferrite and ferrite–semiconductor composites synthesized using biodegradable potato starch displayed remarkable dye degradation activity which can be further explored for photocatalysis application. XRD confirms the pure phases for Fe3O4 (FFO) and ZnO–Fe3O4 (ZFFO), but a secondary SITO phase for TiO2–Fe3O4 (TFFO) and microwave-annealed TiO2–Fe3O4 (TFFO-MWA) due to dynamics between Fe, Ti, and NaOH. FTIR and Raman spectra confirmed the characteristic peaks of the starch surfactant and ferrite and/or semiconducting phase. TEM micrographs demonstrated mixed morphology: spherical particles of size 5–10 nm and rod shape of size 14–67 nm in length and 4–21 nm in diameter. XPS showed different valence states along with satellite peaks besides confirming the elemental composition. BET studies showed type-IV isotherms with H3 adsorption hysteresis indicative of mesoporous structures with ZFFO having the highest surface area (85.9 m2 g−1), minimum pore size, and maximum pore volume, while TFFO-MWA having the lowest surface area (19.1 m2 g−1), maximum pore size, and minimum pore volume. The direct bandgap of FFO, ZFFO, TFFO, and TFFO-MWA samples is found to be 2.50, 2.47, 3.18, and 3.16 eV, respectively. The photocatalytic degradation of MB dye under visible light irradiation was achieved to be 93% in 285 min by FFO, 96% in 150 min by ZFFO, 99% in 180 min by TFFO, and 99% in 90 min by TFFO-MWA. The R2 values of adsorption kinetics studies validated that the kinetics can be best described by the first-order model for FFO, TFFO, TFFO-MWA, and second-order model for ZFFO considering the maximum R2 values being established.












Similar content being viewed by others
References
Valenzuela R (2012) Novel applications of ferrites. Phys Res Int 2012:1–9
Shaikh SF, Ubaidullah M, Mane RS, Al-Enizi AM (2020) Types, synthesis methods and applications of ferrites. Spinel Ferrite Nanostructures for Energy Storage Devices, pp 51–82.
Gupta NK, Ghaffari Y, Kim S, Bae J, Kim KS, Md Saifuddin (2020) Photocatalytic Degradation of Organic Pollutants over MFe2O4 (M= Co, Ni, Cu, Zn) Nanoparticles at Neutral pH. Scientific Reports 10, 4942 (1–11).
Kefeni KK, Mamba BB (2020) Photocatalytic application of spinel ferrite nanoparticles and nanocomposites in wastewater treatment: Review. Sustain Mater Technol 23:e00140.
Zhang F, Wang X, Liu H, Liu C, Wan Y, Long Y, Cai Z (2019) Recent advances and applications of semiconductor photocatalytic technology. Appl Sci 9(12):2489
Siwińska-Stefańska K, Kubiak A, Piasecki A, Goscianska J, Nowaczyk G, Jurga S, Jesionowski T (2018) TiO2-ZnO binary oxide systems: comprehensive characterization and tests of photocatalytic activity. Materials 11(5):841
Zhang F, Wang X, Liu H, Liu C, Wan Y, Long Y, Cai Z (2019) Recent advances and applications of semiconductor photocatalytic technology. Appl Sci 9:2489
M.F. Bellardita, Parrino, E. I. Garcia-Lopez, G. Marci, V. Loddo, L. Palmisano, Heterogeneous photocatalysis for selective formation of high value-added molecules: some chemical and engineering aspects, ACS Catal. 8(12) (2018) 11191–11225.
Zhang Q, Xu X, Liu Y et al (2017) A feasible strategy to balance the crystallinity and specific surface area of metal oxide nanocrystals. Sci Rep 7:46424
Shamsuri AA, Jamil SNAMd (2020) A short review on the effect of surfactants on the Mechanico-Thermal Properties of polymer nanocomposites. Appl Sci 10(14):4867
Klekotka U, Satuła D, Basa A, Kalska-Szostko B (2020) Importance of Surfactant Quantity and Quality on Growth Regime of Iron Oxide Nanoparticles. Materials 13(7):1747
Liang Q, Liu X, Zenga G, Liu Z, Tang L, Shao B, Zeng Z, Zhang W, Liu Y, Cheng M, Tang W, Gong S (2019) Surfactant-assisted synthesis of photocatalysts: Mechanism, synthesis, recent advances and environmental application. Chem Eng J 372:429–451
J. N. Putro, S. Ismadji, C. Gunarto, F. E. Soetaredjo, Y. H. Ju, A study of anionic, cationic, and nonionic surfactants modified starch nanoparticles for hydrophobic drug loading and release, J. Mol. Liq. 298 (2020)112034.
Md. Ibrahim H. Mondal, Md. Mofakkharul Islam, Md. Khademul Islam, Md. Mohsin Hossain, Eco-Friendly Biodegradable Semi-Natural Surfactants from Starch for Green Chemistry, IOSR-JAC. 9(6) (2016) 01–09.
Szymońska J, Molenda M, Wieczorek J (2015) Study of quantitative interactions of potato and corn starch granules with ions in diluted solutions of heavy metal salts. Carbohyd Polym 134:102–109
Shukla A, Bhardwaj AK, Singh S, Uttam K, Gautam N, Himanshu A, Shah J, Kotnala R, Gopal R (2018) Microwave assisted scalable synthesis of titanium ferrite nanomaterials. J Appl Phys 123:161411.
Kumar A, Kuang Y, Liang Z, Sun X (2020) Microwave chemistry, recent advancements and eco-friendly microwave-assisted synthesis of nanoarchitectures and their applications: a review. Mater Today Nano 11:100076.
Kuang Y, Zhang X, Zhou S (2020) Adsorption of methylene blue in water onto activated carbon by surfactant modification. Water 12(2):587
Silva VAJ, Andrade PL, Silva MPC, Bustamante A, Luis De Los Santos Valladares D, Aguiar JA (2013), Synthesis and characterization of Fe3O4 nanoparticles coated with fucan polysaccharides. J Magn Magn Mater 343:138–143.
Fonin M, Pentcheva R, Dedkov, Yu. S., Sperlich M, Vyalikh DV, Scheffler M, Rudiger U, Guntherodt G (2005) Surface electronic structure of the Fe3O4(100): Evidence of a half-metal to metal transition. Phys Rev B 72:104436.
Singh A, Vishwakarma HL (2015) Study of structural, morphological, optical and electroluminescent properties of undoped ZnO nanorods grown by a simple chemical precipitation. MATER SCI-POLAND 33(4):751–759
Guza L, Famá L, Candal R, Goyanes S (2017) Size effect of ZnO nanorods on physicochemical properties of plasticized starch composites. Carbohydr Polym 157:1611–1619
Wang Y-L, Li X-B, Wang B, Zhou Q-S, Qi T-G, Liu G-H, Peng Z-H, Zhou K-C (2019) Interactions of iron and titanium-bearing minerals under high-temperature Bayer digestion conditions. Hydrometallurgy 184:192–198
Sharotri N, Sud D (2015) A greener approach to synthesize visible light responsive nanoporous S-doped TiO2 with enhanced photocatalytic activity. N J Chem 39:2217– 2223.
Díaz de León JN, Rodríguez JR, Rojas J, Esqueda-Barrón Y, Cardenas L, Ramesh Kumar G, Alonso-Nuñez, Fuentes-Moyado S (2019) New Insight on the Formation of Sodium Titanates 1D Nanostructures and Its Application on CO2 Hydrogenation. Front Chem 7:750.
De Vidales JLM, Delgado AL, Vila E, López FA (1999) Effect of the starting solution on the physico-chemical properties of zinc ferrite synthesized at low temperature. J Alloys Comp 287:276–283
Roeinfard M, Bahari A (2017) Nanostructural characterization of the Fe3O4/ZnO magnetic nanocomposite as an application in medicine. A J Supercond Nov Magn 30:3541
Wei JH, Leng CJ, Zhang XZ, Li WH, Liu ZY, Shi J (2009) Synthesis and magnetorheological effect of Fe3O4–TiO2 nanocomposite. J Phys Conf 149:012083.
Tsuji E, Ken-ichi Fukui, Imanishi A (2014) Influence of surface roughening of Rutile Single-Crystalline TiO2 on photocatalytic activity for oxygen photoevolution from water in acidic and alkaline solutions. J Phys Chem C 118 (10):5406–5413.
Nagarajan S, Kuppusamy KA (2013) Extracellular synthesis of zinc oxide nanoparticle using seaweeds of gulf of Mannar, India. J Nanobiotechnol 11:39
Liang Y, He X, Chen L, Zhang Y (2014) Facile preparation of graphene/Fe3O4/TiO2 multifunctional composite for highly selective and sensitive enrichment of phosphopeptides. RSC Adv 4:18132–18135
May M (2015) Eid, spectroscopic characterization of iron oxide nanoparticles functionalized with Chitosan biosynthesis by a clean one pot method. Middle East J Appl Sci 5(5):18–22
Ravindra AV, Chandrika M, Ch. Rajesh, Pratap Kollu, Shaohua Ju, Ramarao SD (2010) Simple synthesis, structural and optical properties of cobalt ferrite nanoparticles. Euro Phys J Plus 134:296.
de Jesus Ruiz-Baltazar A, Reyes-Lopez SY, Perez R (2017) Magnetic structures synthesized by controlled oxidative etching: structural characterization and magnetic behavior. Results Phys 7:1828–1832.
Khan TM, Bibi T, Hussain B (2015) Synthesis and optical study of heat-treated ZnO nanopowder for optoelectronic applications. Bull Mater Sci 38(7):1851–1858
Choi HC, Jung YM, Kim SB (2005) Size effects in the Raman spectra of TiO2 nanoparticles. Vib Spectrosc 37:33–38
Fábio S, Adailton A-F, Mauricelio B, Karla B, Jean-Louis B, Ewerton C, Roberto M, Valder F, Ariete R, Raman P (2018) FTIR, and DFT study of Na2Ti3O7 microcrystals. J RAMAN SPECTROSC 49(3):538–548
Svetlichnyi VA, Shabalina V, Lapin IN, Goncharova DA, Kharlamova TS, Stadnichenko AI (2019) Comparative study of magnetite nanoparticles obtained by pulsed laser ablation in water and air. Appl Surf Sci 467–468:402–410
Choi JS, Lee H-K, An SJ (2015) Synthesis of a graphene v oxide/sodium silicate nanocomposite using sodium silicate solution. RSC Adv 5:38742–38747
Yangjeh H, Gohari MS (2019) Synthesis of magnetically recoverable visible-light-induced photocatalysts by combination of Fe3O4/ZnO with BiOI and polyaniline. Progr Nat Sci Mater Int 29(2):145–155.
Georgios P, Wolfgang SM (2010) X-ray photoelectron spectroscopy of anatase-TiO2 coated carbon nanotubes. Solid State Phenom 162:163–177
Muniandy SS, Mohd Kaus NH, Jiang ZT, Altarawneh M, Lee HL (2017) Green synthesis of mesoporous anatase TiO2 nanoparticles and their photocatalytic activities. RSC Adv 7(76):48083–48094.
Cychosz KA, Thommes M (2018) Progress in the physisorption characterization of nanoporous gas storage materials. Engineering 4:559–566
Amano F, Nogami K, Tanaka M, Ohtani B (2010) Correlation between surface area and photocatalytic activity for Acetaldehyde Decomposition over Bismuth Tungstate particles with a Hierarchical structure. Langmuir 26(10):7174–7180
Gnanasekaran L, Hemamalini R, Rajendran S, Qin J, Yola ML, Atar N, Gracia F (2019) Nanosized Fe3O4 incorporated on a TiO2 surface for the enhanced photocatalytic degradation of organic pollutants. J Mol Liq 287:110967.
Pierpaoli M, Favoni O, Fava G, Ruello ML (2018) A novel method for the combined photocatalytic activity determination and Bandgap estimationn. Methods Protoc 1:22
Dagher S, Soliman A, Ziout A, Tit N, Alnaqbi AH, Khashan S, Alnaimat F, Qudeiri JA (2018) Photocatalytic removal of methylene blue using titania- and silica-coated magnetic nanoparticles. Mater Res Express 5:065518.
Zhou S, Du Z, Li X, Zhang Y, He Y, Zhang Y (2019) Degradation of methylene blue by natural manganese oxides: kinetics and transformation products. R Soc Open Sci 6:190351.
Wang X, Han S, Zhang Q, Zhang N, Zhao D (2018) Photocatalytic oxidation degradation mechanism study of methylene blue dye waste water with GR/iTO2. MATEC Web of Conferences 238:03006
El-Zomrawy AA (2013) Kinetic studies of photoelectrocatalytic degradation of Ponceau 6R dye with ammonium persulfate. J Saudi Chem Soc 17:397–402
López-Vásquez AF, Cobo-Angel MI, Convers-Sánchez JD (2019) Effect of photocatalytic pretreatment of potato starch for bioethanol production using Saccharomyces cerevisiae during simultaneous saccharification-fermentation (SSF). DYNA 86(208):251–256
Lewicka K, Siemion P, Kurcok P (2015) Chemical modifications of starch: microwave effect. Int J Polym Sci 2015:110
Van Hung N, Nguyet BTM, Nghi NH, Khieu DQ (2021) Photocatalytic degradation of Methylene Blue by using ZnO/Longan seed activated carbon under visible-light region. J Inorg Organomet Polym Mater 31:446–459
Xu L, Zheng R, Liu S, Song J, Chen J, Dong B, Song H (2012) NiO@ZnO heterostructured nanotubes: coelectrospinning fabrication, characterization, and highly enhanced gas sensing properties. Inorg Chem 51:7733–7740
Kaneco S, Rahman MA, Suzuki T, Katsumata H, Ohta K (2004) Optimization of solar photocatalytic degradation conditions of bisphenol A in water using titanium dioxide. J Photochem Photobiol A 163:419–424
Mohamed MM, Bayoumy WA, Goher ME, Abdo MH, Mansour El-Ashkar TY (2017) Optimization of-Fe2O3@Fe3O4 incorporated N-TiO2 as super effective photocatalysts under visible light irradiation. Appl Surface Sci 412:668–682.
Govindhan P, Pragathiswaran C, Chinnadurai M (2018) A magnetic Fe3O4 decorated TiO2 nanoparticles application for photocatalytic degradation of methylene blue (MB) under direct sunlight irradiation. J Mater Sci Mater Electron 29:6458
Acknowledgements
M. Chandrika would like to acknowledge the Department of Science and Technology for providing funding through the DST-FIST Level-1 scheme to the Department of Physics, KLEF; File No: SR/FST/PS-1/2018/35. A. V. Ravindra acknowledges the financial support provided by Yunnan Province Post-Doctoral Training Fund-2018 and Kunming University of Science and Technology for the Post-Doctoral Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Handling Editor: Andrea de Camargo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chandrika, M., Ravindra, A.V., Wang, S.Y. et al. Potato starch-assisted green synthesis of nanoferrite and ferrite–semiconductor nanocomposites for effective visible light photocatalytic degradation of methylene blue. J Mater Sci 57, 2610–2626 (2022). https://doi.org/10.1007/s10853-021-06701-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06701-8