Abstract
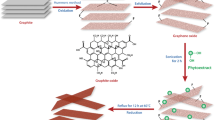
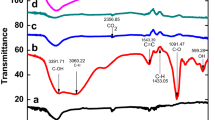
Synthesis of graphene by reducing graphene oxide is the most propitious route for bulk graphene production. Reduction using eco-friendly techniques is more feasible to alleviate toxic chemicals use. Hence, determining the reduction potency of natural substances is vital for further development in the rGO synthesis process. In this work, an experimental investigation was carried out to determine the reduction efficiencies of various natural and thermal reduction techniques. The results were compared with GO reduced with synthetic routes. To ensure accurate determination of reduction potential, constant reaction parameters and the same GO batch synthesized were used. Thorough sample characterization was carried out using FTIR, XPS, FESEM, Raman spectroscopy, XRD and sheet resistance measurements. Results showed that among natural substances, tea powder showed better reduction efficiencies with 13 folds increase in electrical conductivity, whereas synthetic routes showed more than 29 folds increase in the electrical conductivity values as compared to unreduced GO samples. The C/O ratio quantified from XPS analysis was found to be 2.83, 2.98, 3.88 and 6.34 for reduction carried out using lemon extract, coffee, tea powder and direct thermal treatment, respectively. Based on the results, an eco-friendly reduction route using natural substances has the potential for efficient reduction; however, it needs further optimization.
Graphical abstract









Similar content being viewed by others
References
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6:183–191. https://doi.org/10.1038/nmat1849
Randviir EP, Brownson DAC, Banks CE (2014) A decade of graphene research: production, applications and outlook. Mater Today 17:426–432. https://doi.org/10.1016/J.MATTOD.2014.06.001
Yang S, Ricciardulli AG, Liu S et al (2017) Ultrafast delamination of graphite into high-quality graphene using alternating currents. Angew Chem Int Ed 56:6669–6675. https://doi.org/10.1002/anie.201702076
Alam SN, Sharma N, Kumar L (2017) Synthesis of graphene oxide (GO) by modified hummers method and its thermal reduction to obtain reduced graphene oxide (rGO)*. Graphene 06:1–18. https://doi.org/10.4236/graphene.2017.61001
Novoselov KS, Jiang D, Schedin F et al (2005) Two-dimensional atomic crystals. Proc Natl Acad Sci 102:10451–10453. https://doi.org/10.1073/PNAS.0502848102
Chen S, Wu Q, Mishra C et al (2012) Thermal conductivity of isotopically modified graphene. Nat Mater 11:203–207. https://doi.org/10.1038/nmat3207
Pei S, Cheng HM (2012) The reduction of graphene oxide. Carbon 50:3210–3228. https://doi.org/10.1016/j.carbon.2011.11.010
Htwe YZN, Chow WS, Suda Y et al (2019) Effect of electrolytes and sonication times on the formation of graphene using an electrochemical exfoliation process. Appl Surf Sci 469:951–961. https://doi.org/10.1016/j.apsusc.2018.11.029
Gürünlü B, Yücedağ T, Rahim Bayramoğlu M et al (2020) Green synthesis of graphene from graphite in molten salt medium. J Nanomater. https://doi.org/10.1155/2020/7029601
Agarwal V, Zetterlund PB (2021) Strategies for reduction of graphene oxide – a comprehensive review. Chem Eng J 405:127018. https://doi.org/10.1016/j.cej.2020.127018
Shen L, Zhang L, Wang K et al (2018) Analysis of oxidation degree of graphite oxide and chemical structure of corresponding reduced graphite oxide by selecting different-sized original graphite. RSC Adv 8:17209–17217. https://doi.org/10.1039/c8ra01486h
Khan J, Momin SA, Mariatti M (2020) A review on advanced carbon-based thermal interface materials for electronic devices. Carbon 168:65–112. https://doi.org/10.1016/j.carbon.2020.06.012
De Silva KKH, Huang HH, Joshi R, Yoshimura M (2020) Restoration of the graphitic structure by defect repair during the thermal reduction of graphene oxide. Carbon 166:74–90. https://doi.org/10.1016/j.carbon.2020.05.015
Khan J, Mariatti M (2021) The influence of substrate functionalization for enhancing the interfacial bonding between graphene oxide and nonwoven polyester. Fibers Polym. https://doi.org/10.1007/s12221-021-1386-y
Alim MA, Abdullah MZ, Aziz MSA, Kamarudin R (2021) Die attachment, wire bonding, and encapsulation process in LED packaging: a review. Sensors Actuators A Phys 329:112817. https://doi.org/10.1016/j.sna.2021.112817
Abel LL, Levy BB, Brodie BB, Kendall FE (1952) A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J Biol Chem 195:357–366. https://doi.org/10.1016/S0021-9258(19)50907-3
Staudenmaier L (1898) Verfahren zur darstellung der graphitsäure. Ber Dtsch Chem Ges 31:1481–1487. https://doi.org/10.1002/cber.18980310237
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339. https://doi.org/10.1021/ja01539a017
Marcano DC, Kosynkin DV, Berlin JM et al (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814. https://doi.org/10.1021/nn1006368
Mohanapriya K, Ghosh G, Jha N (2016) Solar light reduced Graphene as high energy density supercapacitor and capacitive deionization electrode. Electrochim Acta 209:719–729. https://doi.org/10.1016/j.electacta.2016.03.111
Coros M, Pogacean F, Turza A et al (2020) Green synthesis, characterization and potential application of reduced graphene oxide. Phys E Low-Dimens Syst Nanostruct 119:113971. https://doi.org/10.1016/j.physe.2020.113971
Xu X, Huang D, Cao K et al (2013) Electrochemically reduced graphene oxide multilayer films as efficient counter electrode for dye-sensitized solar cells. Sci Rep 3:1–7. https://doi.org/10.1038/srep01489
Nethravathi C, Rajamathi M (2008) Chemically modified graphene sheets produced by the solvothermal reduction of colloidal dispersions of graphite oxide. Carbon 46:1994–1998. https://doi.org/10.1016/j.carbon.2008.08.013
Gao W, Alemany LB, Ci L, Ajayan PM (2009) New insights into the structure and reduction of graphite oxide. Nat Chem 1:403–408. https://doi.org/10.1038/nchem.281
Wang Y, Shi Z, Yin J (2011) Facile synthesis of soluble graphene via a green reduction of graphene oxide in tea solution and its biocomposites. ACS Appl Mater Interfaces 3:1127–1133. https://doi.org/10.1021/AM1012613
Lee JM, Jeong G, Lee MY et al (2013) Solubilization of chemically reduced graphene oxide using coffee catechol. Chem Lett 42:189–190. https://doi.org/10.1246/CL.2013.189
Seri-Livni O, Saguy C, Horani F et al (2021) Effective reduction of oxygen debris in graphene oxide. Phys Status Solidi Basic Res 258:1–8. https://doi.org/10.1002/pssb.202000505
Rourke JP, Pandey PA, Moore JJ et al (2011) The real graphene oxide revealed: stripping the oxidative debris from the graphene-like sheets. Angew Chem Int Ed 50:3173–3177. https://doi.org/10.1002/anie.201007520
Aliyev E, Filiz V, Khan MM et al (2019) Structural characterization of graphene oxide: Surface functional groups and fractionated oxidative debris. Nanomaterials. https://doi.org/10.3390/nano9081180
Bonanni A, Ambrosi A, Chua CK, Pumera M (2014) Oxidation debris in graphene oxide is responsible for its inherent electroactivity. ACS Nano 8:4197–4204. https://doi.org/10.1021/NN404255Q
Chen W, Yan L, Bangal PR (2010) Preparation of graphene by the rapid and mild thermal reduction of graphene oxide induced by microwaves. Carbon 48:1146–1152. https://doi.org/10.1016/j.carbon.2009.11.037
Lesiak B, Trykowski G, Tóth J et al (2021) Chemical and structural properties of reduced graphene oxide—dependence on the reducing agent. J Mater Sci 56:3738–3754. https://doi.org/10.1007/s10853-020-05461-1
Rabchinskii MK, Dideikin AT, Kirilenko DA et al (2018) Facile reduction of graphene oxide suspensions and films using glass wafers. Sci Rep 8:1–11. https://doi.org/10.1038/s41598-018-32488-x
Wang W, Yu J, Xiang Q, Cheng B (2012) Enhanced photocatalytic activity of hierarchical macro/mesoporous TiO 2-graphene composites for photodegradation of acetone in air. Appl Catal B Environ 119–120:109–116. https://doi.org/10.1016/j.apcatb.2012.02.035
Chen CM, Huang JQ, Zhang Q et al (2012) Annealing a graphene oxide film to produce a free standing high conductive graphene film. Carbon 50:659–667. https://doi.org/10.1016/j.carbon.2011.09.022
Stankovich S, Piner RD, Chen X et al (2006) Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly(sodium 4-styrenesulfonate). J Mater Chem 16:155–158. https://doi.org/10.1039/b512799h
Huang YL, Tien HW, Ma CCM et al (2011) Effect of extended polymer chains on properties of transparent graphene nanosheets conductive film. J Mater Chem 21:18236–18241. https://doi.org/10.1039/c1jm13790e
Pan K, Leng T, Song J et al (2020) Controlled reduction of graphene oxide laminate and its applications for ultra-wideband microwave absorption. Carbon 160:307–316. https://doi.org/10.1016/j.carbon.2019.12.062
Ferrari AC, Robertson J (2000) Interpretation of Raman spectra of disordered and amorphous carbon. Phys Rev B 61:14095. https://doi.org/10.1103/PhysRevB.61.14095
Thakur S, Karak N (2012) Green reduction of graphene oxide by aqueous phytoextracts. Carbon 50:5331–5339. https://doi.org/10.1016/j.carbon.2012.07.023
Hidayah NMS, Liu WW, Lai CW et al (2017) Comparison on graphite, graphene oxide and reduced graphene oxide: synthesis and characterization. AIP Conf Proc. https://doi.org/10.1063/1.5005764
Sánchez-Campos D, Rodríguez-Lugo V, Sánchez-Vargas FC et al (2020) Simple process and uncomplicated reduction of graphene oxide. Mater Chem Phys 242:122325. https://doi.org/10.1016/j.matchemphys.2019.122325
Kumar P, Divya N, Ratan JK (2021) Study on the physico-chemical properties of reduced graphene oxide with different degrees of reduction temperature. J Iran Chem Soc 18:201–211. https://doi.org/10.1007/s13738-020-02014-w
Mishra SK, Tripathi SN, Choudhary V, Gupta BD (2014) SPR based fibre optic ammonia gas sensor utilizing nanocomposite film of PMMA/reduced graphene oxide prepared by in situ polymerization. Sens Actuators B Chem 199:190–200. https://doi.org/10.1016/J.SNB.2014.03.109
Lavin-Lopez MP, Paton-Carrero A, Sanchez-Silva L et al (2017) Influence of the reduction strategy in the synthesis of reduced graphene oxide. Adv Powder Technol 28:3195–3203. https://doi.org/10.1016/j.apt.2017.09.032
Bhuyan MSA, Uddin MN, Islam MM et al (2016) Synthesis of graphene. Int Nano Lett 6:65–83. https://doi.org/10.1007/s40089-015-0176-1
Eckmann A, Felten A, Mishchenko A et al (2012) Probing the nature of defects in graphene by Raman spectroscopy. Nano Lett 12:3925–3930. https://doi.org/10.1021/NL300901A
Luo D, Zhang G, Liu J, Sun X (2011) Evaluation criteria for reduced graphene oxide. J Phys Chem C 115:11327–11335. https://doi.org/10.1021/JP110001Y
Guex LG, Sacchi B, Peuvot KF et al (2017) Experimental review: chemical reduction of graphene oxide (GO) to reduced graphene oxide (rGO) by aqueous chemistry. Nanoscale 9:9562–9571. https://doi.org/10.1039/c7nr02943h
Kuang B, Song W, Ning M et al (2018) Chemical reduction dependent dielectric properties and dielectric loss mechanism of reduced graphene oxide. Carbon 127:209–217. https://doi.org/10.1016/j.carbon.2017.10.092
Cobos M, González B, Fernández MJ, Fernández MD (2018) Study on the effect of graphene and glycerol plasticizer on the properties of chitosan-graphene nanocomposites via in situ green chemical reduction of graphene oxide. Int J Biol Macromol 114:599–613. https://doi.org/10.1016/J.IJBIOMAC.2018.03.129
Akhavan O, Kalaee M, Alavi ZS et al (2012) Increasing the antioxidant activity of green tea polyphenols in the presence of iron for the reduction of graphene oxide. Carbon 50:3015–3025. https://doi.org/10.1016/j.carbon.2012.02.087
Weng X, Wu J, Ma L et al (2019) Impact of synthesis conditions on Pb(II) removal efficiency from aqueous solution by green tea extract reduced graphene oxide. Chem Eng J 359:976–981. https://doi.org/10.1016/j.cej.2018.11.089
Bo Z, Shuai X, Mao S et al (2014) Green preparation of reduced graphene oxide for sensing and energy storage applications. Sci Rep 4:1–8. https://doi.org/10.1038/srep04684
Vu THT, Tran TTT, Le HNT et al (2015) A new green approach for the reduction of graphene oxide nanosheets using caffeine. Bull Mater Sci 38:667–671. https://doi.org/10.1007/s12034-015-0896-x
Zhao B, Liu P, Jiang Y et al (2012) Supercapacitor performances of thermally reduced graphene oxide. J Power Sources 198:423–427. https://doi.org/10.1016/j.jpowsour.2011.09.074
Acknowledgements
The authors would like to express their gratitude to Universiti Sains Malaysia for providing us with a University Research Grant (No:8014044).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare the following financial interests/personal relationships, which may be considered as potential competing interests.
Additional information
Handling Editor: Christopher Blanford.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, J., Jaafar, M. Reduction efficiencies of natural substances for reduced graphene oxide synthesis. J Mater Sci 56, 18477–18492 (2021). https://doi.org/10.1007/s10853-021-06492-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06492-y