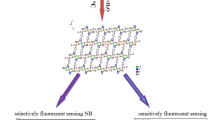
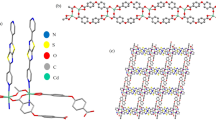
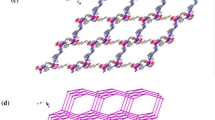
Abstract
The structural and photophysical properties of the [Cd2(H2L)2(H2O)5].5H2O (where H4L is the ligand 5,5'-((thiophene-2,5-dicarbonyl)bis(azanediyl))diisophthalic acid labeled as Cd-MOF), as well as the elucidation of the selective turn-off luminescent sensing mechanism toward 4-nitroaniline (pNA) were addressed, using quantum chemical methods. To reach this aim, the structures of the ground state (S0) and first excited state (S1) Cd-MOF/analyte system were assessed. We found that after the interaction a photoinduced electron transfer (PET) from the Cd-MOF to pNA is responsible for the fluorescence quenching in this system. For this purpose, a study was performed based on TD-DFT and multireference calculations to corroborate that an excited state exists with the adequate electronic configuration for PET process in the interacting system Cd-MOF/analyte. Intermolecular interaction between the Cd-MOF and analyte was studied by means of Morokuma–Ziegler energy decomposition analysis, natural orbitals of chemical valence, ab initio molecular dynamics (AIMD) calculations and non-covalent interactions (NCI) index. These results showed that intermolecular interactions via hydrogen bond are considerably strengthened in the excited state for the Cd-MOF/pNA, which favor the non-radiative deactivation channels of the chemosensor. In addition, the overlap of absorption spectra of Cd-MOF and pNA indicates that the loss of fluorescence is also due to internal filter effect (IFE). The most noteworthy aspect of this methodology is to consider the relative energies of the S0 and S1 states of MOF/analyte system to explaining the experimental behavior of Cd-MOF toward 4-nitroaniline, proving to be a robust tool in the accurate elucidation of the sensing mechanism in the MOF chemosensor.
Graphical abstract

Ab initio methods along with TD-DFT have been used to elucidate the selective turn-off luminescent sensing mechanism of nitroaromatic compounds by a Cd-based metal–organic framework (MOF). The role of the host–guest interaction has been pointed out using different theoretical descriptors. A theoretical protocol is given to get more insights into the MOF design and selectivity for nitroaromatic compounds.










Similar content being viewed by others
References
Ju K, Parales RE (2010) Nitroaromatic compounds, from synthesis to biodegradation. Microbiol Mol Biol Rev 74:250–272. https://doi.org/10.1128/MMBR.00006-10
Bagheri M, Masoomi MY, Morsali A, Schoedel A (2016) Two dimensional host-guest metal-organic framework sensor with high selectivity and sensitivity to picric acid. ACS Appl Mater Interfaces 8:21472–21479. https://doi.org/10.1021/acsami.6b06955
Viola R, Liberatore N, Luciani D, Mengali S (2016) Quartz enhanced photoacoustic spectroscopy for detection of improvised explosive devices and precursors. Adv Opt Technol. https://doi.org/10.1155/2016/5757361
Sun X, Wang Y, Lei Y (2015) Fluorescence based explosives detection: from mechanisms to sensory materials. Chem Soc Rev 44:8019–8061. https://doi.org/10.1039/x0xx00000x
Tiwari J, Tarale P, Sivanesan S, Bafana A (2019) Environmental persistence, hazard, and mitigation challenges of nitroaromatic compounds. Environ Sci Pollut Res 26:28650–28667. https://doi.org/10.1007/s11356-019-06043-8
Gole B, Bar AK, Mukherjee PS (2014) Modification of extended open frameworks with fluorescent tags for sensing explosives: competition between size selectivity and electron deficiency. Chem A Eur J 20:2276–2291. https://doi.org/10.1002/chem.201302455
Du JL, Gao JP, Li CP et al (2017) A stable 3D Cd(II) metal-organic framework for highly sensitive detection of Cu2+ ions and nitroaromatic explosives. RSC Adv 7:49618–49625. https://doi.org/10.1039/c7ra08977e
Karikalan N, Kubendhiran S, Chen SM et al (2017) Electrocatalytic reduction of nitroaromatic compounds by activated graphite sheets in the presence of atmospheric oxygen molecules. J Catal 356:43–52. https://doi.org/10.1016/j.jcat.2017.09.012
Wu D, Sedgwick AC, Gunnlaugsson T et al (2017) Fluorescent chemosensors: the past, present and future. Chem Soc Rev 46:7105–7123
Ma X, Tao F, Zhang Y et al (2017) Detection of nitroaromatic explosives by a 3D hyperbranched σ–π conjugated polymer based on a POSS scaffold. J Mater Chem A 5:14343–14354. https://doi.org/10.1039/C7TA04351A
Hu Z, Deibert BJ, Li J (2014) Luminescent metal-organic frameworks for chemical sensing and explosive detection. Chem Soc Rev 43:5815–5840
Cui Y, Yue D, Huang Y et al (2019) Photo-induced electron transfer in a metal-organic framework: a new approach towards a highly sensitive luminescent probe for Fe3+. Chem Commun 55:11231–11234. https://doi.org/10.1039/c9cc05019a
Hidalgo-Rosa Y, Treto-Suárez MA, Schott E et al (2020) Sensing mechanism elucidation of a chemosensor based on a metal-organic framework selective to explosive aromatic compounds. Int J Quantum Chem . https://doi.org/10.1002/qua.26404
Hidalgo-Rosa Y, Treto-Suarez MA, Schott E et al (2020) Sensing mechanism elucidation of a europium(III) metal-organic framework selective to aniline: a theoretical insight by means of multiconfigurational calculations. J Comput Chem 41:1956–1964. https://doi.org/10.1002/jcc.26365
Lustig WP, Mukherjee S, Rudd ND et al (2017) Metal–organic frameworks: functional luminescent and photonic materials for sensing applications. Chem Soc Rev 46:3242–3285. https://doi.org/10.1039/C6CS00930A
Hu Z, Deibert BJ, Li J (2014) Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem Soc Rev 43:5815–5840. https://doi.org/10.1039/C4CS00010B
Kwok RTK, Leung CWT, Lam JWY, Tang BZ (2015) Biosensing by luminogens with aggregation-induced emission characteristics. Chem Soc Rev 44:4228–4238. https://doi.org/10.1039/c4cs00325j
Cui Y, Zhang J, He H, Qian G (2018) Photonic functional metal-organic frameworks. Chem Soc Rev 47:5740–5785. https://doi.org/10.1039/c7cs00879a
Liu JQ, Luo ZD, Pan Y et al (2020) Recent developments in luminescent coordination polymers: designing strategies, sensing application and theoretical evidences. Coord Chem Rev 406:213145. https://doi.org/10.1016/j.ccr.2019.213145
Lu L, Liu W, Wang J et al (2019) Four new luminescent-organic frameworks exhibiting highly sensing of nitroaromatics: an experimental and computational insight. Inorganica Chim Acta 487:257–263. https://doi.org/10.1016/j.ica.2018.12.013
Dong B-X, Pan Y-M, Liu W-L, Teng Y-L (2017) An ultrastable luminescent metal-organic framework for selective sensing of nitroaromatic compounds and nitroimidazole-based drug molecules. Cryst Growth Des 18:431–440. https://doi.org/10.1021/acs.cgd.7b01430
Hu Z, Qiao C, Xia Z et al (2020) A luminescent mg-metal-organic framework for sustained release of 5-fluorouracil: appropriate host-guest interaction and satisfied acid-base resistance. ACS Appl Mater Interfaces 12:14914–14923. https://doi.org/10.1021/acsami.0c01198
Osman DI, El-Sheikh SM, Sheta SM et al (2019) Nucleic acids biosensors based on metal-organic framework (MOF): Paving the way to clinical laboratory diagnosis. Biosens Bioelectron 141:111451. https://doi.org/10.1016/j.bios.2019.111451
Rowsell JLC, Yaghi OM (2005) Strategies for hydrogen storage in metal-organic frameworks. Angew Chemie Int Ed 44:4670–4679. https://doi.org/10.1002/anie.200462786
Yan D, Tang Y, Lin H, Wang D (2014) Tunable two-color luminescence and host-guest energy transfer of fluorescent chromophores encapsulated in metal-organic frameworks. Sci Rep 4:4337. https://doi.org/10.1038/srep04337
Guajardo-Maturana R, Zarate X, Claveria-Cadiz F, Schott E (2016) A silver coordination cage assembled from [Ag2(bis(isoxazolyl))3]: DFT approach to the binding forces within the host–guest interactions. RSC Adv 6:103346–103356. https://doi.org/10.1039/C6RA22905K
Kasha M (1950) Characterization of electronic transitions in complex molecules. Discuss Faraday Soc 9:14–19
Treto-Suárez MA, Hidalgo-Rosa Y, Schott E et al (2019) Understanding the selective-sensing mechanism of Al3+ cation by a chemical sensor based on schiff base: a theoretical approach. J Phys Chem A 123:6970–6977. https://doi.org/10.1021/acs.jpca.9b03366
Treto-Suárez MA, Hidalgo-Rosa Y, Schott E et al (2019) Radiative decay channel assessment to understand the sensing mechanism of a fluorescent turn-on Al3+ chemosensor. Int J Quantum Chem 120:26083. https://doi.org/10.1002/qua.26083
Wang F, Yu Z, Wang C et al (2017) A multifunctional metal-organic framework showing excellent fluorescence sensing and sensitization. Sens Actuators B Chem 239:688–695. https://doi.org/10.1016/j.snb.2016.08.067
Poater J, Gimferrer M, Poater A (2018) Covalent and ionic capacity of MOFs to sorb small gas molecules. Inorg Chem 57:6981–6990. https://doi.org/10.1021/acs.inorgchem.8b00670
Lee C, Yang W, Parr RG (1988) Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Becke AD (1993) Density-functional thermochemistry III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Neese F (2012) The ORCA program system, Wiley Interdiscip. Comput Mol Sci 2:73–78
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys Chem Chem Phys 7:3297–3305. https://doi.org/10.1039/b508541a
Sjoberg P, Politzer P (1990) Use of the electrostatic potential at the molecular surface to interpret and predict nucleophilic processes. J Phys Chem 94:3959–3961. https://doi.org/10.1021/j100373a017
Barone V, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A 102:1995–2001. https://doi.org/10.1021/jp9716997
Mohan N, Suresh CH (2014) A molecular electrostatic potential analysis of hydrogen, halogen, and dihydrogen bonds. J Phys Chem A 118:1697–1705. https://doi.org/10.1021/jp4115699
Ziegler T, Rauk A (1977) Calculation of bonding energies by hartree-fock slater method. 1 transition-state method. Theor Chim Acta 46:1–10. https://doi.org/10.1007/BF02401406
Kitaura K, Morokuma K (1976) A new energy decomposition scheme for molecular interactions within the Hartree-Fock approximation. Int J Quantum Chem 10:325–340. https://doi.org/10.1002/qua.560100211
Baerends EJ, Ziegler T, Autschbach J, Bashford D, Bérces A, Bickelhaupt FM, Bo C, Boerrigter PM, Cavallo L, Chong DP et al. (2017) ADF2017, SCM, Theoretical Chemistry ( Vrije Universiteit, Amsterdam, The Netherlands). https://www.scm.com
Mitoraj MP (2011) Bonding in ammonia borane: an analysis based on the natural orbitals for chemical valence and the extended transition state method (ETS-NOCV). J Phys Chem A 115:14708–14716. https://doi.org/10.1021/jp209712s
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys . https://doi.org/10.1063/1.3382344
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566. https://doi.org/10.1080/00268977000101561
Contreras-García J, Johnson ER, Keinan S et al (2011) NCIPLOT: a program for plotting noncovalent interaction regions. J Chem Theory Comput 7:625–632. https://doi.org/10.1021/ct100641a
Johnson ER, Keinan S, Mori-Sánchez P et al (2010) Revealing noncovalent interactions. J Am Chem Soc 132:6498–6506. https://doi.org/10.1021/ja100936w
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38
Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K (2005) Scalable molecular dynamics with NAMD. J Comput Chem 26:1781–1802. https://doi.org/10.1002/jcc.20289
Treto-Suárez MA, Tapia J, Hidalgo-Rosa Y et al (2020) New sensitive and selective chemical sensors for Ni2+and Cu2+Ions: insights into the sensing mechanism through DFT methods. J Phys Chem A 124:6493–6503. https://doi.org/10.1021/acs.jpca.0c03834
Zhang X, Chi L, Ji S et al (2009) Rational design of d-PeT phenylethynylated-carbazole monoboronic acid fluorescent sensors for the selective detection of α-hydroxyl carboxylic acids and monosaccharides. J Am Chem Soc 131:17452–17463. https://doi.org/10.1021/ja9060646
Shi J, Izquierdo MA, Oh S et al (2019) Inverted energy gap law for the nonradiative decay in fluorescent floppy molecules: larger fluorescence quantum yields for smaller energy gaps. Org Chem Front 6:1948–1954. https://doi.org/10.1039/c9qo00259f
Shi J, Aguilar Suarez LE, Yoon SJ et al (2017) Solid state luminescence enhancement in π-conjugated materials: unraveling the mechanism beyond the framework of AIE/AIEE. J Phys Chem C 121:23166–23183. https://doi.org/10.1021/acs.jpcc.7b08060
Roos BO, Taylor PR, Sigbahn PEM (1980) A complete active space SCF method (CASSCF) using a density matrix formulated super-CI approach. Chem Phys 48:157–173. https://doi.org/10.1016/0301-0104(80)80045-0
Angeli C, Cimiraglia R, Evangelisti S et al (2001) Introduction of n-electron valence states for multireference perturbation theory. J Chem Phys 114:10252. https://doi.org/10.1063/1.1361246
Bentzien J, Muller RP, Floria J, Warshel A (1998) Hybrid ab initio quantum mechanics / molecular mechanics calculations of free energy surfaces for enzymatic reactions: the nucleophilic attack in subtilisin. J Phys Chem B 102:2293–2301. https://doi.org/10.1021/jp973480y
Carloni P, Rothlisberger U, Parrinello M (2002) The role and perspective of Ab initio molecular dynamics in the study of biological systems. Acc Chem Res 35:455–464. https://doi.org/10.1021/ar010018u
Biczók L, Bérces T, Linschitz H (1997) Quenching processes in hydrogen-bonded pairs: interactions of excited fluorenone with alcohols and phenols. J Am Chem Soc 119:11071–11077. https://doi.org/10.1021/ja972071c
Inoue H, Hida M, Nakashima N, Yoshihara K (1982) Picosecond fluorescence lifetimes of anthraquinone derivatives. Radiationless deactivation via intra- and intermolecular hydrogen bonds. J Phys Chem 86:3184–3188. https://doi.org/10.1021/j100213a024
Politzer P, Murray JS (1991) Molecular electrostatic potentials and chemical reactivity. Rev Comput Chem. https://doi.org/10.1002/chin.200427290
Mitoraj MP, Michalak A (2013) Theoretical description of halogen bonding—An insight based on the natural orbitals for chemical valence combined with the extended-transition- state method (ETS-NOCV). J Mol Model 19:4681–4688. https://doi.org/10.1007/s00894-012-1474-4
Berrones-Reyes J, Muñoz-Flores BM, Gómez-Treviño A et al (2019) Novel fluorescent schiff bases as Al3+ sensors with high selectivity and sensitivity, and their bioimaging applications. Mater Chem Phys 233:89–101. https://doi.org/10.1016/j.matchemphys.2019.05.035
Berrones-Reyes JC, Muñoz-Flores BM, Cantón-Diáz AM et al (2019) Quantum chemical elucidation of the turn-on luminescence mechanism in two new Schiff bases as selective chemosensors of Zn2+: synthesis, theory and bioimaging applications. RSC Adv 9:30778–30789. https://doi.org/10.1039/c9ra05010h
Zhao Z, Song X, Liu L et al (2018) A recognition mechanism study: Luminescent metal-organic framework for the detection of nitro-explosives. J Mol Graph Model 80:132–137. https://doi.org/10.1016/j.jmgm.2017.12.024
Wang P, Song X, Zhao Z et al (2016) Role of the electronic excited-state hydrogen bonding in the nitro-explosives detection by [Zn2(oba)2(bpy)]. Chem Phys Lett 661:257–262. https://doi.org/10.1016/j.cplett.2016.06.085
Zhao G-J, Han K-L (2007) Ultrafast hydrogen bond strengthening of the photoexcited fluorenone in alcohols for facilitating the fluorescence quenching†. J Phys Chem A 111:9218–9223. https://doi.org/10.1021/jp0719659
Flom SR, Barbara PF (1985) Proton transfer and hydrogen bonding in the internal conversion of S1 anthraquinones. J Phys Chem 89:4489–4494. https://doi.org/10.1021/j100267a017
Fan L, Wang F, Zhao D et al (2020) Two cadmium(II) coordination polymers as multi-functional luminescent sensors for the detection of Cr(VI) anions, dichloronitroaniline pesticide, and nitrofuran antibiotic in aqueous media. Spectrochim Acta - Part A Mol Biomol Spectrosc 239:118467. https://doi.org/10.1016/j.saa.2020.118467
Fan L, Wang F, Zhao D et al (2020) A self-penetrating and chemically stable zinc (ii)-organic framework as multi-responsive chemo-sensor to detect pesticide and antibiotics in water. Appl Organomet Chem. https://doi.org/10.1002/aoc.5960
Chen S, Yu YL, Wang JH (2018) Inner filter effect-based fluorescent sensing systems: a review. Anal Chim Acta 999:13–26. https://doi.org/10.1016/j.aca.2017.10.026
Panigrahi SK, Mishra AK (2019) Inner filter effect in fluorescence spectroscopy: as a problem and as a solution. J Photochem Photobiol C Photochem Rev 41:100318. https://doi.org/10.1016/j.jphotochemrev.2019.100318
Kozich V, Werncke W, Dreyer J et al (2002) Vibrational excitation and energy redistribution after ultrafast internal conversion in 4-nitroaniline. J Chem Phys 117:719–726. https://doi.org/10.1063/1.1482698
Kovalenko SA, Farztdinov VM, Schanz R, et al (2000) Femtosecond relaxation of photoexcited p-nitroaniline in water: solvation internal conversion and cooling. Conference Quantum Electron Laser Sci - Tech Dig Ser 195
Natali M, Campagna S, Scandola F (2014) Photoinduced electron transfer across molecular bridges: electron- and hole-transfer superexchange pathways. Chem Soc Rev 43:4005–4018. https://doi.org/10.1039/C3CS60463B
Chen Y, Tsao K, Keillor JW (2015) Fluorogenic protein labelling: a review of photophysical quench mechanisms and principles of fluorogen design. Can J Chem 93:389–398. https://doi.org/10.1139/cjc-2014-0405
Feng Y, Cheng J, Zhou L et al (2012) Ratiometric optical oxygen sensing: a review in respect of material design. Analyst 137:4885. https://doi.org/10.1039/c2an35907c
Treto-Suárez MA, Hidalgo-Rosa Y, Schott E et al (2020) Fluorescence turn-on and turn-off mechanisms of a dual-selective chemosensor of Bi3+ and Ph changes: insights from a theoretical perspective. Dye Pigment .https://doi.org/10.1016/j.dyepig.2020.108934
Liu YH, Zhao GJ, Li GY, Han KL (2010) Fluorescence quenching phenomena facilitated by excited-state hydrogen bond strengthening for fluorenone derivatives in alcohols. J Photochem Photobiol A Chem 209:181–185. https://doi.org/10.1016/j.jphotochem.2009.11.012
Chandra A (2003) Dynamical behavior of anion−water and water−water hydrogen bonds in aqueous electrolyte solutions: a molecular dynamics study. J Phys Chem B 107:3899–3906. https://doi.org/10.1021/jp022147d
Naberukhin YI, Voloshin VP (2009) Distributions of hydrogen bond lifetimes in instantaneous and inherent structures of water. Zeitschrift fur Phys Chemie 223:1119–1131. https://doi.org/10.1524/zpch.2009.6062
Acknowledgements
The authors thank PhD Program in Molecular Physical Chemistry from University Andres Bello, a subsidy of the DAD-UNAB, FONDECYT 1180565, FONDECYT 1180017, FONDECYT 1201880, FONDECYT Iniciación Grant No 11180650 and ANID-Postdoctoral 3210271. This work was funded by ANID—Millennium Science Initiative Program—NCN17_040. ANID/FONDAP/15110019.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Handling Editor: Pedro Camargo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hidalgo-Rosa, Y., Mena-Ulecia, K., Treto-Suárez, M.A. et al. Insights into the selective sensing mechanism of a luminescent Cd(II)-based MOF chemosensor toward NACs: roles of the host–guest interactions and PET processes. J Mater Sci 56, 13684–13704 (2021). https://doi.org/10.1007/s10853-021-06196-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06196-3