Abstract
In this work, PtCo nanoparticles (NPs) on hierarchically ordered mesoporous carbon (PtCo/OMC) are synthesized for polymer electrolyte membrane fuel cells (PEMFCs) aiming to improve the activity and durability of the Pt-based catalyst towards oxygen reduction reaction (ORR). Specifically, the OMC is prepared through a solvent evaporation-induced self-assembly (EISA) method by using a triblock copolymer PEO-PPO-PEO as the structure agent, followed by annealing in the nitrogen atmosphere to decompose the structure agent and to carbonize the carbon precursor. PtCo nanoparticles (NPs) are fabricated with an average diameter of 3.3 nm by H2 reduction and galvanic replacement in an acid solution, and then are uniformly dispersed onto the OMC via impregnation. The typical mesoporous structure of the OMC enhances the uniformly distribution and thermal stability of the small-sized PtCo NPs. The activity and the durability of the as-prepared PtCo/OMC catalyst are investigated by cyclic voltammetry (CV) and single-cell test. In the electrochemical tests, PtCo/OMC exhibits a high ECSA value of 88.56 m2g−1, and a ECSA retention of 77.5% after 5000 CV cycles. The results show that the PtCo/OMC catalyst is more active and more stable than the commercial-carbon-supported PtCo catalyst (PtCo/XC-72).
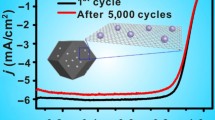
Graphical abstract
PtCo nanoparticles (NPs) on hierarchically ordered mesoporous carbon (PtCo/OMC) are synthesized aiming to improve the activity and stability of the Pt-based catalyst for the oxygen reduction reaction (ORR). Due to the natural mesoporous and graphite-carbon nanostructure of the OMC, the as-prepared PtCo/OMC catalyst is more active and more stable than the PtCo NPs on the commercial Vulcan@ XC-72 support (PtCo/XC-72), showing great potential in proton exchange membrane fuel cells (PEMFCs).








Similar content being viewed by others
References
Tian XL, Zhao X, Su YQ, Wang LJ, Wang HM, Dang D, Chi B, Liu HF, Hensen EJM, Lou XW, Xia BY (2019) Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science 366:850–856
Gan Y, Wang ZD, Shi Y, Guo CQ, Tan HY, Lu ZX, Yan CF (2018) Synthesis of density-multiplied Pt-NP arrays and their application in fuel cell by self-assembly of di-block copolymer. Electrochim Acta 283:1–10
Wee J-H (2007) Applications of proton exchange membrane fuel cell systems. Renew Sust Energy Rev 11:1720–1738
Thomas JM, Edwards PP, Dobson PJ, Owen GP (2020) Decarbonising energy: The developing international activity in hydrogen technologies and fuel cells. J Energ Chem 51:405–415
Ryoo R, Kim J, Jo C, Han SW, Kim JC, Park H, Han J, Shin HS, Shin JW (2020) Rare-earth-platinum alloy nanoparticles in mesoporous zeolite for catalysis. Nature 585:221–224
Wang Z-D, Gan Y, Mai YL, Shi Y, Cao S, Lu Z-X, Guo C-Q, Tan H-Y, Yan C-Q (2020) Synthesis of ordered Pt Nanocube arrays directed by block copolymer nanotemplate and their potential on ethanol oxidation reaction. Anal Chem 92:8046–8050
Biz C, Fianchini M, Polo V, Gracia J (2020) Magnetism and heterogeneous catalysis: in depth on the quantum spin-exchange interactions in Pt3M (M = V, Cr, Mn, Fe Co, Ni, and Y) (111) alloys. ACS Appl Mater Interfaces 12:50484–50494
Chaisubanan N, Maniwan W, Hunsom M (2017) Effect of heat-treatment on the performance of PtM/C (M = Cr, Pd, Co) catalysts towards the oxygen reduction reaction in PEM fuel cell. Energy 127:454–461
Zhao X, Chen S, Fang Z, Ding J, Sang W, Wang Y, Zhao J, Peng Z, Zeng J (2015) Octahedral Pd@Pt1.8Ni core-shell nanocrystals with ultrathin PtNi alloy shells as active catalysts for oxygen reduction reaction. J Am Chem Soc 137:2804–2807
Mani P, Srivastava R, Strasser P (2011) Dealloyed binary PtM3 (M=Cu Co, Ni) and ternary PtNi3M (M=Cu Co, Fe, Cr) electrocatalysts for the oxygen reduction reaction: Performance in polymer electrolyte membrane fuel cells. J Power Sources 196:666–673
Li Y, Wang F, Zhu H (2020) Synthesis of H2O2–CTAB dual-modified carbon black-supported Pt3Ni to improve catalytic activity for ORR. J Mater Sci 55:11241–11252. https://doi.org/10.1007/s10853-020-04808-y
Yang S, Zhang F, Gao C, Xia J, Lu L, Wang Z (2017) A sandwich-like PtCo-graphene/carbon dots/graphene catalyst for efficient methanol oxidation. J Electroanal Chem 802:27–32https://doi.org/10.1016/j.jelechem.2017.08.027
Liu M, He S, Chen W (2016) Free-standing 3d hierarchical carbon foam-supported ptco nanowires with “Pt skin” as advanced electrocatalysts. Electrochim Acta 199:218–226.
Liu X, Zhang M, Yang T, Wang L, Zhu H, Wang S, Du M (2016) Carbon nanofibers as nanoreactors in the construction of PtCo alloy carbon core-shell structures for highly efficient and stable water splitting. Mater Des 109:162–170
Xiong Y, Yang Y, DiSalvo FJ, Abruna HD (2020) Synergistic bimetallic metallic organic framework-derived pt-co oxygen reduction electrocatalysts. ACS Nano 14:13069–13080
Oezaslan M, Hasché F, Strasser P (2012) Oxygen electroreduction on ptco3, ptco and pt3co alloy nanoparticles for alkaline and acidic PEM fuel cells. J Electrochem Soc 159:B394–B405
Antolini E (2003) Formation, microstructural characteristics and stability of carbon supported platinum catalysts for low temperature fuel cells. J Mater Sci 38:2995–3005. https://doi.org/10.1023/A:1024771618027
Wang YJ, Zhao N, Fang B, Li H, Bi XT, Wang H (2015) Carbon-supported Pt-based alloy electrocatalysts for the oxygen reduction reaction in polymer electrolyte membrane fuel cells: particle size, shape, and composition manipulation and their impact to activity. Chem Rev 115:3433–3467. https://doi.org/10.1021/cr500519c
Zhao HB, Li AW, Gu JH, Xiong GX (1999) A novel preparation method for porous noble metal/ceramic catalytic membranes. J Mater Sci 34:2987–2996 https://doi.org/10.1023/A:1004616309085
Liu Y, Wang Z, Teng W, Zhu H, Wang J, Elzatahry AA, Al-Dahyan D, Li W, Deng Y, Zhao D (2018) A template-catalyzed in situ polymerization and co-assembly strategy for rich nitrogen-doped mesoporous carbon. J Mater Chem A 6:3162–3170
Zhang Y, Yue Q, Yu L, Yang X, Hou XF, Zhao D, Cheng X, Deng Y (2018) Amphiphilic block copolymers directed interface coassembly to construct multifunctional microspheres with magnetic core and monolayer mesoporous aluminosilicate shell. Adv Mater 30:1800345–1800354
Wang C, Zhao Y, Zhou L, Liu Y, Zhang W, Zhao Z, Hozzein WN, Alharbi HMS, Li W, Zhao D (2018) Mesoporous carbon matrix confinement synthesis of ultrasmall WO3 nanocrystals for lithium ion batteries. J Mater Chem A 6:21550–21557
Onoe T, Iwamoto S, Inoue M (2007) Synthesis and activity of the Pt catalyst supported on CNT. Catal Commun 8:701–706
Hyeon T, Han S, Sung YE, Park KW, Kim YW (2003) High-performance direct methanol fuel cell electrodes using solid-phase-synthesized carbon nanocoils. Angew Chem Int Ed Engl 42:4352–4356
Park K-W, Sung Y-E (2004) Origin of the enhanced catalytic activity of carbon nanocoil-supported PtRu alloy electrocatalysts. J Phys Chem B 108:939–944
Chai GS, Yoon SB, Kim JH, Yu JS (2004) Spherical carbon capsules with hollow macroporous core and mesoporous shell structures as a highly efficient catalyst support in the direct methanol fuel cell. Chem Commun (Camb) 23:2766–2767
Liang C, Li Z, Dai S (2008) Mesoporous carbon materials: synthesis and modification. Angew Chem Int Ed Engl 47:3696–3717
Szczęśniak B, Choma J, Jaroniec M (2018) Effect of graphene oxide on the adsorption properties of ordered mesoporous carbons toward H2, C6H6, CH4 and CO2. Microporous Mesoporous Mater 261:105–110
Léonard AF, Castro-Muñiz A, Suárez-García F, Job N, Paredes JI (2020) Understanding the effect of the mesopore volume of ordered mesoporous carbons on their electrochemical behavior as Li-ion battery anodes. Microporous Mesoporous Mater 306:110417–110442
Joseph S, Kempaiah DM, Benzigar MR, Ilbeygi H, Singh G, Talapaneni SN, Park D-H, Vinu A (2019) Highly ordered mesoporous carbons with high specific surface area from carbonated soft drink for supercapacitor application. Microporous Mesoporous Mater 280:337–346
Yang Y, Gao G, Zhang X, Li F (2014) Facile fabrication of composition-tuned Ru–Ni bimetallics in ordered mesoporous carbon for levulinic acid hydrogenation. ACS Catal 4:1419–1425
Zhou J-H, He J-P, Ji Y-J, Dang W-J, Liu X-L, Zhao G-W, Zhang C-X, Zhao J-S, Fu Q-B, Hu H-P (2007) CTAB assisted microwave synthesis of ordered mesoporous carbon supported Pt nanoparticles for hydrogen electro-oxidation. Electrochim Acta 52:4691–4695
Raghuveer V, Manthiram A (2005) Mesoporous carbons with controlled porosity as an electrocatalytic support for methanol oxidation. J Electrochem Soc 152:A1504–A1510
Xie M, Dong H, Zhang D, Guo X, Ding W (2011) Simple synthesis of highly ordered mesoporous carbon by self-assembly of phenol–formaldehyde and block copolymers under designed aqueous basic/acidic conditions. Carbon 49:2459–2464
Gu D, Meng Y, Zhang F, Shi Y, Cheng L, Feng D, Wu Z, Chen Z, Wan Y, Stein A, Zhao D (2006) A family of highly ordered mesoporous polymer resin and carbon structures from organic-organic self-assembly. Chem Mater 18:4447–4464
Ma TY, Liu L, Yuan ZY (2013) Direct synthesis of ordered mesoporous carbons. Chem Soc Rev 42:3977–4003
Kuan YD, Ke TR, Lyu JL, Sung MF, Do JS (2020) Development of a current collector with a graphene thin film for a proton exchange membrane fuel cell module. Molecules 25:955–971
Liu SH, Lu RF, Huang SJ, Lo AY, Chien SH, Liu SB (2006) Controlled synthesis of highly dispersed platinum nanoparticles in ordered mesoporous carbons. Chem Commun (Camb) 32:3435–3437
Lu AH, Spliethoff B, Schüth F (2008) Aqueous synthesis of ordered mesoporous carbon via self-assembly catalyzed by amino acid. Chem Mater 20:5314–5319
Liang C, Wang X, Dai S (2008) Facile synthesis of ordered mesoporous carbons with high thermal stability by self-assembly of resorcinol-formaldehyde and block copolymers under highly acidic conditions. Langmuir 24:7500–7505
Li J, Zhang Y, Tian C, Zhou H, Hu G, Xia R (2020) Structurally ordered nanoporous Pt–Co alloys with enhanced mechanical behaviors in tension. Microporous Mesoporous Mater 295:109955–109964
Shellard PM, Srisubin T, Hartmann M, Butcher J, Fei F, Cox H, McNamara TP, McArdle T, Shepherd AM, Jacobs RMJ, Waigh TA, Flitscht SL, Blanford CF (2020) A versatile route to edge-specific modifications to pristine graphene by electrophilic aromatic substitution. J Mater Sci 55:10284–10302 https://doi.org/10.1007/s10853-020-04662-y
Shukla AK, Aricòa AS, Kimc H, Parkc S, Minc M, Antonuccia V (2001) An XPS study on oxidation states of Pt and its alloys with Co and Cr and its relevance to electroreduction of oxygen. Appl Surf Sci 172:33–40
He C, Zhang S, Tao J, Shen PK (2018) One-step solid state synthesis of PtCo nanocubes/graphene nanocomposites as advanced oxygen reduction reaction electrocatalysts. J Catal 362:85–93
Yan ZX, Gao LN, Dai CJ, Zhang MM, Lv XM, Shen PK (2018) Metal-free mesoporous carbon with higher contents of active N and S codoping by template method for superior ORR efficiency to Pt/C. Int J Hydrog Energy 43:3705–3715
Jia RP, Gan ZZ, Huang H, Sheng ZM (2021) Controlled synthesis of mesoporous carbon with ultra-high N-doping structure from polymer precursor for efficient electrocatalysis of oxygen reduction. Electrochim Acta 368:137617–137624
Gong L, Sun J, Li XD, Huang B, Yang GC, Liu YS (2021) One-step and controllable synthesis of active N-rich graphene nanoclusters-CNT composite via an ultrafast deflagration reaction for oxygen reduction electrocatalysis. J Mater Sci 56:6349–6360. https://doi.org/10.1007/s10853-020-05671-7
Acknowledgements
The authors are grateful for DNL Cooperation Fund of CAS (DNL180405), Natural Science Foundation of Guangdong Province (2015A030312007, 2017A030310539 and 2018A050506071), Guangzhou Science and Technology Project (201904010412, 202002030349), and STS Regional Key Project of Chinese Academy of Sciences (KFJ-STS-QYZD-2021-02-003). And with special thanks to Mr. Liang Zheng and Ms. Wang Xiaoman for the TEM and EDS mapping technique.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Christopher Blanford.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, Y., Wang, Z., Mai, Y. et al. Highly active PtCo nanoparticles on hierarchically ordered mesoporous carbon support for polymer electrolyte membrane fuel cells. J Mater Sci 56, 13083–13095 (2021). https://doi.org/10.1007/s10853-021-06159-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06159-8