Abstract
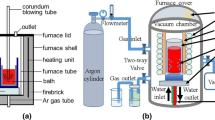
Reactions of metallurgical-grade silicon (MG-Si) with gaseous hydrogen bromide (HBr) has been monitored by means of online gas chromatography in a flow reactor system. The formation of tri-bromosilane started to occur at 380 °C, accompanied by the consumption of HBr. The conversion of HBr into bromosilanes increased with an increase in reaction temperature and reached a maximum at 440 °C. An increase of the HBr concentration in a HBr-N2 mixed gas led to an increase in the consumption of MG-Si, while it reduced the selectivity of the tri-bromosilane formation. An increase in total flow rate of the reaction gas caused a dramatic decrease in the HBr conversion and enhanced the selectivity of the tri-bromosilane formation. The rate constant for overall bromination reaction at 400 °C was measured to be 0.46 s−1. Concentrations of impurities in the product were much less than those in MG-Si. Moreover, kerf loss silicon was subjected to the bromination reaction under the optimized conditions.





Similar content being viewed by others
References
Chapin DM, Fuller CS, Pearson GL (1954) J Appl Phys 25:676
Kamerski LL (2006) J Electron Spectrosc 150:105
Coletti G, Bronsveld PCP, Hahn G, Warta W, Macdonald D, Ceccaroli B, Wambach K, Quang NL, Fernandez JM (2011) Adv Funct Mater 21:879
Pizzini S (2009) Appl Phys A 96:171
Braga AFB, Moreira SP, Zampieri PR, Bacchin JMG, Mei PR (2008) Sol Eng Mater Sol Cells 92:418
Woditsch P, Koch W (2002) Sol Eng Mater Sol Cells 72:11
Sarti D, Einhaus R (2002) Sol Eng Mater Sol Cells 72:27
Moller HJ (2004) Adv Eng Mater 6:501
Dhamrin M, Saitoh T, Kamisako K, Mori T, Iwamoto N (2010) In: Proceedings of the 25th European Photovoltaic Solar Energy Conference and Exhibition/5th World Conference on Photovoltaic Energy Conversion, Valencia, Spain, pp 1600–1603
Pires JCS, Otubo J, Braga AFB, Mei PR (2005) J Mater Process Technol 169:16
Wang TY, Lin YC, Tai CY, Sivakumar R, Rai DK, Lan CW (2008) J Crystal Growth 310:3403
Mukashev BN, Abdullin KA, Tamendarov MF, Turmagambetov TS, Beketov BA, Page MR, Kline DM (2009) Sol Eng Mater Sol Cells 93:1785
Martinez AM, Osen KS, Kongstein OE, Sheridan E, Ulyashin A, Haarberg GM (2010) ECS Trans 25:107
Lin YC, Wang TY, Lan CW, Tai CY (2010) Powder Technol 200:216
Kong MS, Jung HC, Hong HS, Kim GS, Chung HS (2011) Current Appl Phys 11:554
Uesawa N, Shen P, Inasawa S, Miyoshi A, Yamaguchi Y (2011) Chem Eng J 114:4291
Schumacher J, Woerner L, Moore E, Newman C (1979) Final Report, D.O.E./J.P.L. Contract no. 954,914, John C. Schumacher Co., USA. This work was performed for the Jet Propulsion Laboratory, California Institute of Technology by agreement between NASA and D.O.E
Schumb WC, Young RC (1930) J Am Chem Soc 52:1464
Chase MW Jr, Davies CA, Downey JR Jr, Frurip DJ, McDonald RA, Syverud AN (1985) JANAF thermochemical tables, vol 14, 3rd edn. J Phys Chem Ref Data Suppl 1
Ingel WM, Peffley MS (1985) J Electrochem Soc 1236
Levenspiel O (1999) In: Anderso W, Santor K (eds) Chemical reaction engineering, Chap 5, 3rd edn. Wiley, New York, pp 101–110
Rodriguez H, Guerrero I, Koch W, Endros AL, Franke D, Haßler C, Kalejs JP, Moller HJ (2011) In: Luque A, Hegedus S (eds) Handbook of photovoltaic science and engineering, Chap 6, 2nd edn. Wiley, New York
Acknowledgements
This research was supported by Yamaguchi Green Materials Cluster Initiative from the Ministry of Education, Culture, Sports, Science and Technology, Japan. We are very grateful to Prof. S. Sakuragi, Prof. Y. Sakata, and Prof. M. Yoshimoto for their comments and advices.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tomono, K., Okamura, Y., Furuya, H. et al. Selective hydrobromination of metallurgical-grade silicon in a flow reactor system. J Mater Sci 47, 3227–3232 (2012). https://doi.org/10.1007/s10853-011-6160-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-011-6160-x