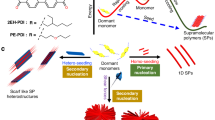
Abstract
A novel [c2] daisy chain was successfully constructed by the hermaphroditic monomer of dibenzo[24]-crown-8 (DB24C8) derivative bearing secondary ammonium salt (1) from the analysis of the solution-phase behavior of parent monomers and single-crystal X-ray analysis. 1H NMR spectroscopy was employed to show that the crown ether moiety and the secondary ammonium salt unit underwent acid–base and alkali metal cation dependent switches. The complexation behavior of this hermaphroditic monomer in the solution was further demonstrated to exhibit the controlled photophysical behavior as a reversible luminescent switch in the presence of acids or bases. Solid morphology was determined by SEM.











Similar content being viewed by others
References
Fernando, I.R., Bairu, S.G., Ramakrishna, G., Mezei, G.: Single-color pseudorotaxane-based temperature sensing. New J. Chem. 34, 2097–2100 (2010). https://doi.org/10.1039/C0NJ00541J
Li, Y.-P., Yang, H.-R., Zhao, Q., Song, W.-C., Han, J., Bu, X.-H.: Ratiometric and selective fluorescent sensor for Zn2+ as an “Off− On−Off” switch and logic gate. Inorg. Chem. 51, 9642–9648 (2012). https://doi.org/10.1021/ic300738e
Bren, V.A.: Fluorescent and photochromic chemosensors. Rus. Chem. Rev. 70, 1017–1036 (2001). https://doi.org/10.1070/RC2001v070n12ABEH000667
Lin, T.-C., Lai, C.-C., Chiu, S.-H.: A guanidinium ion-based anion- and solvent polarity-controllable molecular switch. Org. Lett. 11, 613–616 (2009). https://doi.org/10.1021/ol802638k
Asakawa, M., Iqbal, S., Stoddart, J.F., Tinker, N.D.: Prototype of an optically responsive molecular switch based on pseudorotaxane, Angew. Chem. Int. Ed. 35, 976–978 (1996). https://doi.org/10.1002/anie.199609761
Muraoka, M., Irie, H., Nakatsuji, Y.: Acid/base controllable molecular switch based on a neutral phenanthroline guest penetrated pseudorotaxane. Org. Bio. Chem. 8, 2408–2413 (2010). https://doi.org/10.1039/B926010B
Hidekazu, M., Simon, R.C., Ivan, P., Tucker, J.H.R.: A ditopic ferrocene receptor for anions and cations that functions as a chromogenic molecular switch. Chem. Commun. 64–65 (2003). https://doi.org/10.1039/B210227G
Caroline, C., Camille, R., Emile, B., Frederic, C.: A pH-sensitive lasso-based rotaxane molecular switch. Chem. Eur. J. 19, 2982–2989 (2013). https://doi.org/10.1002/chem.201203597
Cheng, H.-B., Zhang, H.-Y., Liu, Y.: Dual-stimulus luminescent lanthanide molecular switch based on an unsymmetrical diarylperfluorocyclopentene. J. Am. Chem. Soc. 135, 10190–10193 (2013). https://doi.org/10.1021/ja4018804
Sapna, S., Gregory, J.E.D., Stephen, J.L.: Controlling the ON/OFF threading of a terpyridine containing [2]pseudorotaxane ligand via changes in coordination geometry. Chem. Commun. (2008). https://doi.org/10.1039/B716117D
Tokunaga, Y., Nakamura, T., Yoshioka, M., Shimomura, Y.: A molecular switch based on acid and base promoted, cation governed binding in a crown ether threaded rotaxane. Tetra. Lett. 47, 5901–5904 (2006). https://doi.org/10.1016/j.tetlet.2006.06.062
Coutrot, F., Romuald, C., Busseron, E.: A new pH-switchable dimannosyl[c2]daisy chain molecular machine. Org. Lett. 10, 3741–3744 (2008). https://doi.org/10.1021/ol801390h
Li, J.J., Zhao, F., Li, J.: Polyrotaxanes for applications in life science and biotechnology. Appl. Microbiol. Biotechnol. 90, 427–443 (2011). https://doi.org/10.1007/s00253-010-3037-x
Dong, S., Luo, Y., Yan, X., Zheng, B., Ding, X., Yu, Y., Ma, Z., Zhao, Q., Huang, F.: A dual-responsive supramolecular polymer gel formed by crown ether based molecular recognition. Angew. Chem. Int. Ed. 50, 1905–1909 (2011). https://doi.org/10.1002/anie.201006999
Bissell, R.A., Córdova, E., Kaifer, A.E., Stoddart, J.F.: A chemically and electrochemically switchable molecular shuttle. Nature. 369, 133–137 (1994). https://doi.org/10.1038/369133a0
Jeppesen, J.O., Vignon, S.A., Stoddart, J.F.: In the twilight zone between [2]pseudorotaxanes and [2]rotaxanes. Chem. Eur. J. 9, 4611–4625 (2003). https://doi.org/10.1002/chem.200304798
Vignon, S.A., Jarrosson, T., Iijima, T., Tseng, H.-R., Sanders, J.K.M.: Stoddart: switchable neutral bistable rotaxanes. J. Am. Chem. Soc. 126, 9884–9885 (2004). https://doi.org/10.1021/ja048080k
Crowley, J.D., Leigh, D.A., Lusby, P.J., McBurney, R.T., Perret-Aebi, L.-E., Petzold, C., Slawin, A.M.Z., Symes, M.D.: Switchable palladium-complexed molecular shuttle and its metastable positional isomers. J. Am. Chem. Soc. 129, 15085–15090 (2007). https://doi.org/10.1021/ja076570h
Crowley, J.D., Hanni, K.D., Leigh, D.A., Slawin, A.M.Z.: Diels−Alder active-template synthesis of rotaxanes and metal-ion-switchable molecular shuttles. J. Am. Chem. Soc. 132, 5309–5314 (2010). https://doi.org/10.1021/ja101029u
Wang, X., Zhu, J., Smithrud, D.B.: Synthesis and investigation of host-[2]rotaxanes that bind metal cations. J. Org. Chem. 75, 3358–3370 (2010). https://doi.org/10.1021/jo100330e
Badji′c, J.D., Balzani, V., Credi, A., Silvi, S., Stoddart, J.F.: A molecular elevator. Science. 303, 1845–1849 (2004). https://doi.org/10.1126/science.1094791
Badji′c, J.D., Ronconi, C.M., Stoddart, J.F., Balzani, V., Silvi, S., Credi, A.: Operating molecular elevators. J. Am. Chem. Soc. 128, 1489–1499 (2006). https://doi.org/10.1021/ja0543954
Jiang, Q., Zhang, H.-Y., Han, M., Ding, Z.-J., Liu, Y.: pH-controlled intramolecular charge-transfer behavior in bistable [3]Rotaxane. Org. Lett. 12, 1728–1731 (2010). https://doi.org/10.1021/ol100321k
Keaveney, C.M., Leigh, D.A.: Shuttling through anion recognition. Angew. Chem Int. Ed. 43, 1222–1224 (2004). https://doi.org/10.1002/anie.200353248
Serreli, V., Lee, C.-F., Kay, E.R., Leigh, D.A.: A molecular information ratchet. Nature. 445, 523–527 (2007). https://doi.org/10.1038/nature05452
Saha, S., Stoddart, J.F.: Photo-driven molecular devices. Chem. Soc. Rev. 36, 77–92 (2007). https://doi.org/10.1039/B607187B
Coskun, A., Friedman, D.C., Li, H., Patel, K., Khatib, H.A., Stoddart, J.F.: A Light-gated stop−go molecular shuttle. J. Am. Chem. Soc. 131, 2493–2495 (2009). https://doi.org/10.1021/ja809225e
Li, S.J., Taura, D., Hashidzume, A., Harada, A.: Light-switchable janus [2]rotaxanes based on α-cyclodextrin derivatives bearing two recognition sites linked with oligo(ethylene glycol). Chem. Asian J. 5, 2281–2289 (2010). https://doi.org/10.1002/asia.201000169
Balzani, V., Credi, A., Venturi, M.: Light powered molecular machines. Chem. Soc. Rev. 38, 1542–1550 (2009). https://doi.org/10.1039/B806328C
Wang, C., Olson, M.A., Fang, L., Ben’itez, D., Tkatchouk, E., Basu, S., Basuray, A.N., Zhang, D., Zhu, D., Goddard, W.A., Stoddart, J.F.: Isolation by crystallization of translational isomers of a bistable donor-acceptor [2]catenane. Proc. Natl. Acad. Sci. U. S. A. 107, 13991–13996 (2010). https://doi.org/10.1073/pnas.100930210
Farrell, A.A., Kay, E.R., Bottari, G., Leigh, D.A., Jarvis, S.P.: The effect of solvent upon molecularly thin rotaxane film formation. Appl. Surf. Sci. 253, 6090–6095 (2007). https://doi.org/10.1016/j.apsusc.2007.01.006
Ashton, P.R., Baxter, I., Cantrill, S.J., Fyfe, M.C.T., Glink, P.T., Stoddart, J.F., White, A.J.P., Williams, D.J.: Supramolecular daisy chains Angew. Chem Int. Ed. 37, 1294–1297 (1998). https://doi.org/10.1002/(SICI)1521-3773
Cantrill, S.J., Youn, G.J., Stoddart, J.F., Williams, D.J.: Supramolecular daisy chains. J. Org. Chem. 66, 6857–6872 (2001). https://doi.org/10.1021/jo010405h
Fang, L., Hmadeh, M., Wu, J., Olson, M.A., Spruell, J.M., Trabolsi, A., Yang, Y.-W., Elhabiri, M., Albrecht-Gary, A.-M., Stoddart, J.F.: Acid−base actuation of [c2]daisy chains. J. Am. Chem. Soc. 131, 7126–7134 (2009). https://doi.org/10.1021/ja900859d
Hmadeh, M., Fang, L., Trabolsi, A., Elhabiri, M., Albrecht- Gary, A.-M., Stoddart, J.F.: On the thermodynamic and kinetic investigations of a [c2]daisy chain polymer. J. Mater. Chem. 20, 3422–3430 (2010). https://doi.org/10.1039/B924273B
Yamaguchi, N., Nagvekar, D.S., Gibson, H.W.: Self-organization of a heteroditopic molecule to linear polymolecular arrays in solution. Angew. Chem Int. Ed. 37, 2361–2364 (1998). https://doi.org/10.1002/(SICI)1521-3773(19980918)
Wu, J., Leung, K.C.-F., Beni’tez, D., Han, J.-Y., Cantrill, S.J., Fang, L., Stoddart, J.F.: An acid–base-controllable [c2]daisy Chain. Angew. Chem. Int. Ed. 47, 7470–7474 (2008). https://doi.org/10.1002/anie.200803036
Zheng, B., Zhang, M., Dong, S., Liu, J., Huang, F.-H.: A benzo-21-crown-7/secondary ammonium salt [c2]Daisy Chain. Org. Lett. 14, 306–309 (2012). https://doi.org/10.1021/ol203062w
Badjic, J.D., Ronconi, C.M., Stoddart, J.F., Balzani, V., Silvi, S., Credi, A.: Operating molecular elevators. J. Am. Chem. Soc. 128, 1489–1499 (2006). https://doi.org/10.1021/ja0543954
Nguyen, T., Tseng, H.-R., Celestre, P.C., Flood, A.H., Liu, Y., Zink, J.I., Stoddart, J.F.: A reversible molecular valve. Proc. Natl. Acad. Sci. U. S. A. 102, 10029–10034 (2005). https://doi.org/10.1073/pnas.0504109102
Ashton, P.R., Balzani, V., Kocian, O., Prodi, L., Spencer, N., Stoddart, J.F.: A light-fueled, “piston cylinder” molecular-level machine. J. Am. Chem. Soc. 120, 11190–11191 (1998). https://doi.org/10.1021/ja981889a
Credi, A.: Artificial molecular motors powered by light. Aust. J. Chem. 59, 157–169 (2006). https://doi.org/10.1071/CH06025
Collin, J.P., Heitz, V., Bonnet, S., Sauvage, J.P.: Transition metal-complexed catenanes and rotaxanes in motion: Towards molecular machines. Inorg. Chem. Commun. 8, 1063–1074 (2005). https://doi.org/10.1016/j.inoche.2005.07.016
Chuang, C.-J., Li, W.-S., Lai, C.-C., Liu, Y.-H., Peng, S.-M., Chao, I., Chiu, S.-H.: A molecular cage-based [2]rotaxane that behaves as a molecular muscle. Org. Lett. 11, 385–388 (2009). https://doi.org/10.1021/ol802648h
Jimene, M.C., Buchecker, C.D., Sauvage, J.P.: Towards synthetic molecular muscles: contraction and stretching of a linear rotaxane dimer. Angew. Chem Int. Ed. 39, 3284–3287 (2000)
Takeda, Y.: Thermodynamic study for dibenzo-24-crown-8 complexes with Alkali metal ions in nonaqueous solvents. Bull. Chem. Soc. Jpn 56, 3600–3602 (1983). https://doi.org/10.1246/bcsj.56.3600
Takeda, Y., Kudo, Y., Fujiwara, S.: Thermodynamic study for complexation reactions of dibenzo-24-crown-8 with alkali metal ions in acetonitrile. Bull. Chem. Soc. Jpn 58, 1315–1316 (1985). https://doi.org/10.1246/bcsj.58.1315
Tawarah, K.M., Mizyed, S.A.: A conductance study of the association of alkali cations with 1,13-dibenzo-24-crown-8 in acetonitrile. J. Solut. Chem. 18, 387–401 (1989). https://doi.org/10.1007/BF00656776
Frensdorff, H.K.: Stability constants of cyclic polyether complexes with univalent cations. J. Am. Chem. Soc. 93, 600–606 (1971). https://doi.org/10.1021/ja00732a007
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) (Grant No. 21978067), Natural Science Foundation of Hebei Province (Grant No. C2021208024, B2020208085) and Program of Introducing Talents and Wisdom of Hebei Province.
Author information
Authors and Affiliations
Contributions
Xia Tian ,shengwei Zhou, Yupeng Wang , Yuting Li, Chengbin Wang, Wei Su , these authors participated in the preparation, separation and property testing of the experimental compounds in the thesis. Jianrong Han and Shouxin Liu were involved in the writing and revision of the manuscript 。
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tian, X., Han, J., Zhou, S.w. et al. Chemically controlled self-assembly behaviors of dibenzo-24-crown-8 bearing ammonium salt moiety. J Incl Phenom Macrocycl Chem 103, 441–450 (2023). https://doi.org/10.1007/s10847-023-01208-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-023-01208-y