Abstract
Purpose
What is the rate of euploidy and clinical viability of embryos resulting from micro 3 pronuclei zygotes?
Methods
Retrospective cohort analysis in a single, academic in vitro fertilization (IVF) center from March 2018 to June 2021. Cohorts were separated by fertilization as either a 2 pronuclear zygote (2PN) or micro 3 pronuclear zygote (micro 3PN). PGT-A was performed to identify embryonic ploidy rates in embryos created from micro 3PN zygotes. The clinical outcomes of all transferred euploid micro 3PN zygotes were evaluated from frozen embryo transfer (FET) cycles.
Results
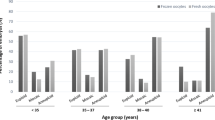
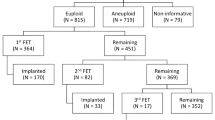
During the designated study period, 75,903 mature oocytes were retrieved and underwent ICSI. Of these, 60,161 were fertilized as 2PN zygotes (79.3%) and 183 fertilized as micro 3PN zygotes (0.24%). Of the micro 3PN-derived embryos that underwent biopsy, 27.5% (n=11/42) were deemed euploid by PGT-A, compared to 51.4% (n=12,301/23,923) of 2PN-derived embryos, p=0.06. Four micro 3PN-derived embryos were transferred in subsequent single euploid FET cycles, which includes one live birth and one ongoing pregnancy.
Conclusion
Micro 3PN zygotes that develop to the blastocyst stage and meet the criteria for embryo biopsy have the potential to be euploid by preimplantation genetic testing for aneuploidy (PGT-A) and if selected for transfer can achieve a live birth. Although there are a significantly lower number of micro 3PN embryos that make it to blastocyst biopsy, the potential to continue to culture abnormally fertilized oocytes may give these patients a chance at pregnancy that they previously did not have.


Similar content being viewed by others
References
Alpha Scientists in Reproductive Medicine and European Society for Human Reproduction and Embryology Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–83.
Nagy ZP, Janssenswillen C, Janssens R, De Vos A, Staessen C, Van de Velde H, Van Steirteghem AC. Timing of oocyte activation, pronucleus formation and cleavage in humans after intracytoplasmic sperm injection (ICSI) with testicular spermatozoa and after ICSI or in-vitro fertilization on sibling oocytes with ejaculated spermatozoa. Hum Reprod. 1998 ;13(6):1606–12. https://doi.org/10.1093/humrep/13.6.1606.
Capalbo A, Treff N, Cimadomo D, Tao X, Ferrero S, Vaiarelli A, Colamaria S, Maggiulli R, Orlando G, Scarica C, Scott R, Ubaldi FM, Rienzi L. Abnormally fertilized oocytes can result in healthy live births: improved genetic technologies for preimplantation genetic testing can be used to rescue viable embryos in in vitro fertilization cycles. Fertil Steril. 2017;108(6):1007–1015.e3. https://doi.org/10.1016/j.fertnstert.2017.08.004.
Plachot M, de Grouchy J, Junca AM, Mandelbaum J, Salat-Baroux J, Cohen J. Chromosome analysis of human oocytes and embryos: does delayed fertilization increase chromosome imbalance? Hum Reprod. 1988 ;3(1):125–7. https://doi.org/10.1093/oxfordjournals.humrep.a136644.
Rosenbusch B. The chromosomal constitution of embryos arising from monopronuclear oocytes in programmes of assisted reproduction. Int J Reprod Med. 2014;2014:418198.
Staessen C, Van Steirteghem AC. The chromosomal constitution of embryos developing from abnormally fertilized oocytes after intracytoplasmic sperm injection and conventional in-vitro fertilization. Hum Reprod. 1997;12(2):321–7. https://doi.org/10.1093/humrep/12.2.321.
Feenan K, Herbert M. Can ‘abnormally’ fertilized zygotes give rise to viable embryos? Hum Fertil (Camb). 2006;9(3):157–69. https://doi.org/10.1080/14647270600636269.
Munné S, Tang YX, Grifo J, Cohen J. Origin of single pronucleated human zygotes. J Assist Reprod Genet. 1993;10(4):276–9. https://doi.org/10.1007/BF01204942.
Chen X, Shi S, Mao J, Zou L, Yu K. Developmental potential of abnormally fertilized oocytes and the associated clinical outcomes. Front Physiol. 2020;4:528424. https://doi.org/10.3389/fphys.2020.528424.
Gras L, Trounson AO. Pregnancy and birth resulting from transfer of a blastocyst observed to have one pronucleus at the time of examination for fertilization. Hum Reprod. 1999;14(7):1869–71. https://doi.org/10.1093/humrep/14.7.1869.
Itoi F, Asano Y, Shimizu M, Honnma H, Murata Y. Birth of nine normal healthy babies following transfer of blastocysts derived from human single-pronucleate zygotes. J Assist Reprod Genet. 2015 ;32(9):1401–7. https://doi.org/10.1007/s10815-015-0518-y.
Macas E, Imthurn B, Roselli M, Keller PJ. Chromosome analysis of single- and multipronucleated human zygotes proceeded after the intracytoplasmic sperm injection procedure. J Assist Reprod Genet. 1996;13(4):345–50. https://doi.org/10.1007/BF02070150.
Mateo S, Parriego M, Boada M, Vidal F, Coroleu B, Veiga A. In vitro development and chromosome constitution of embryos derived from monopronucleated zygotes after intracytoplasmic sperm injection. Fertil Steril. 2013;99(3):897–902.e1. https://doi.org/10.1016/j.fertnstert.2012.11.014. Epub 2012 Dec 14
Mateo S, Vidal F, Parriego M, Rodríguez I, Montalvo V, Veiga A, Boada M. Could monopronucleated ICSI zygotes be considered for transfer? Analysis through time-lapse monitoring and PGS. J Assist Reprod Genet. 2017;34(7):905–11. https://doi.org/10.1007/s10815-017-0937-z.
van der Heijden GW, van den Berg IM, Baart EB, Derijck AA, Martini E, de Boer P. Parental origin of chromatin in human monopronuclear zygotes revealed by asymmetric histone methylation patterns, differs between IVF and ICSI. Mol Reprod Dev. 2009;76(1):101–8. https://doi.org/10.1002/mrd.20933.
Flaherty SP, Payne D, Swann NJ, Matthews CD. Assessment of fertilization failure and abnormal fertilization after intracytoplasmic sperm injection (ICSI). Reprod Fertil Dev. 1995;7(2):197–210. https://doi.org/10.1071/rd9950197.
Sachs AR, Politch JA, Jackson KV, Racowsky C, Hornstein MD, Ginsburg ES. Factors associated with the formation of triploid zygotes after intracytoplasmic sperm injection. Fertil Steril. 2000;73(6):1109–14. https://doi.org/10.1016/s0015-0282(00)00521-5.
Ezoe K, Takahashi T, Shimazaki K, Miki T, Tanimura Y, Amagai A, Sawado A, Akaike H, Mogi M, Kaneko S, Kato M, Kato K, Tarozzi N, Borini A, Coticchio G. Human 1PN and 3PN zygotes recapitulate all morphokinetic events of normal fertilization but reveal novel developmental errors. Hum Reprod. 2022;37(10):2307–19. https://doi.org/10.1093/humrep/deac177.
Li M, Zhao W, Xue X, Zhang S, Shi W, Shi J. Three pro-nuclei (3PN) incidence factors and clinical outcomes: a retrospective study from the fresh embryo transfer of in vitro fertilization with donor sperm (IVF-D). Int. J Clin Exp Med. 2015;8(8):13997–4003.
Mutia K, Wiweko B, Iffanolida PA, Febri RR, Muna N, Riayati O, Jasirwan SO, Yuningsih T, Mansyur E, Hestiantoro A. The frequency of chromosomal euploidy among 3PN embryos. J Reprod Infertil. 2019;20(3):127–31.
Gu C, Li K, Li R, Li L, Li X, Dai X, He Y. Chromosomal aneuploidy associated with clinical characteristics of pregnancy loss. Front Genet. 2021;15(12):667697. https://doi.org/10.3389/fgene.2021.667697.
Grau N, Escrich L, Martín J, Rubio C, Pellicer A, Escribá MJ. Self-correction in tripronucleated human embryos. Fertil Steril. 2011;96:951–6.
Joergensen MW, Labouriau R, Hindkjaer J, Stougaard M, Kolevraa S, Bolund L, Agerholm IE, Sunde L. The parental origin correlates with the karyotype of human embryos developing from tripronuclear zygotes. Clin Exp Reprod Med. 2015 ;42(1):14–21. https://doi.org/10.5653/cerm.2015.42.1.14.
Takahashi H, Hirata R, Otsuki J, Habara T, Hayashi N. Are tri-pronuclear embryos that show two normal-sized pronuclei and additional smaller pronuclei useful for embryo transfer? Reprod Med Biol. 2022;21(1):e12462. https://doi.org/10.1002/rmb2.12462.
Rodriguez-Purata J, Lee J, Whitehouse M, Duke M, Grunfeld L, Sandler B, Copperman A, Mukherjee T. Reproductive outcome is optimized by genomic embryo screening, vitrification, and subsequent transfer into a prepared synchronous endometrium. J Assist Reprod Genet. 2016;33(3):401–12. https://doi.org/10.1007/s10815-016-0647-y.
Nazem TG, Sekhon L, Lee JA, Overbey J, Pan S, Duke M, Briton-Jones C, Whitehouse M, Copperman AB, Stein DE. The correlation between morphology and implantation of euploid human blastocysts. Reprod Biomed Online. 2019;38(2):169–76. https://doi.org/10.1016/j.rbmo.2018.10.007.
Hernandez-Nieto C, Lee JA, Slifkin R, Sandler B, Copperman AB, Flisser E. What is the reproductive potential of day 7 euploid embryos? Hum Reprod. 2019;34(9):1697–706. https://doi.org/10.1093/humrep/dez129.
Hernandez-Nieto C, Lee JA, Alkon-Meadows T, Luna-Rojas M, Mukherjee T, Copperman AB, Sandler B. Late follicular phase progesterone elevation during ovarian stimulation is not associated with decreased implantation of chromosomally screened embryos in thaw cycles. Hum Reprod. 2020;35(8):1889–99. https://doi.org/10.1093/humrep/deaa123.
Walters-Sen L, Neitzel D, Bristow SL, Mitchell A, Alouf CA, Aradhya S, Faulkner N. Experience analysing over 190,000 embryo trophectoderm biopsies using a novel FAST-SeqS preimplantation genetic testing assay. Reprod Biomed Online. 2022;44(2):228–38. https://doi.org/10.1016/j.rbmo.2021.06.022.
Barak Y, Kogosowski A, Goldman S, Soffer Y, Gonen Y, Tesarik J. Pregnancy and birth after transfer of embryos that developed from single-nucleated zygotes obtained by injection of round spermatids into oocytes. Fertil Steril. 1998;70(1):67–70. https://doi.org/10.1016/s0015-0282(98)00106-x.
Chen Z, Yan J, Feng HL. Aneuploid analysis of tripronuclear zygotes derived from in vitro fertilization and intracytoplasmic sperm injection in humans. Fertil Steril. 2005;83(6):1845–8. https://doi.org/10.1016/j.fertnstert.2004.11.076.
Kola I, Trounson A, Dawson G, Rogers P. Tripronuclear human oocytes: altered cleavage patterns and subsequent karyotypic analysis of embryos. Biol Reprod. 1987;37(2):395–401. https://doi.org/10.1095/biolreprod37.2.395.
Yalçınkaya E, Özay A, Ergin EG, Öztel Z, Özörnek H. Live birth after transfer of a tripronuclear embryo: an intracytoplasmic sperm injection as a combination of microarray and time-lapse technology. Turk. J Obstet Gynecol. 2016;13(2):95–8. https://doi.org/10.4274/tjod.45144.
Acknowledgements
The authors thank all the physicians, fellows, embryologists, and research and staff members for the valuable work and help in the realizing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB# 18-00441) of the Icahn School of Medicine at Mount Sinai approved this study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
Alan Copperman is a board member of Sema4 Genomics and Progyny and possesses stock/stock options in Sema4 Genomics and Progyny. The other coauthors declare no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Canon, C., Thurman, A., Li, A. et al. Assessing the clinical viability of micro 3 pronuclei zygotes. J Assist Reprod Genet 40, 1765–1772 (2023). https://doi.org/10.1007/s10815-023-02830-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-02830-y