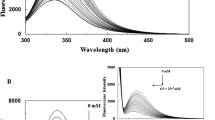
The interaction of multi-walled carbon nanotubes (MWCNTs) with catalase is investigated using fluorescence and circular dichroism spectroscopic techniques. The results of the fluorescence experiments suggest that MWCNTs quench the intrinsic fluorescence of catalase via a static quenching mechanism. The circular dichroism spectral results reveal the unfolding of catalase with a significant decrease in the α-helix content in the presence of MWCNTs, which indicates that the conformation of catalase is changed in the binding process, thereby remarkably decreasing its activity. The binding constants and the number of binding sites of the MWCNT to the catalase are calculated at different temperatures. The thermodynamic parameters, such as the changes in free energy (ΔG), enthalpy (ΔH), and entropy (ΔS), are calculated using thermodynamic equations. The fact that all negative values of ΔG, ΔH, and ΔS are obtained suggests that the interaction of the MWCNTs with catalase is spontaneous, and that hydrogen bonding and van der Waals interactions play an important role in the binding process.
Similar content being viewed by others
References
X. Li, F. Zhang, and D. Zhao, Nano Today, 8, No. 6, 643–676 (2013).
C. Cha, S. R. Shin, N. Annabi, M. R. Dokmeci, and A. Khademhosseini, ACS Nano, 7, No. 4, 2891–2897 (2013).
K. Song, Y. Zhang, J. Meng, E. C. Green, N. Tajaddod, H. Li, and M. L. Minus, Materials, 6, No. 6, 2543–2577 (2013).
B. T. Zhang, X. Zheng, H. F. Li, and J. M. Lin, Anal. Chim. Acta, 784, 1–17 (2013).
S. Park, M. Vosguerichian, and Z. Bao, Nanoscale, 5, No. 5, 1727–1752 (2013).
S. Y. Madani, A. Mandel, and A. M. Seifalian, Nano Rev., 4, 21521 (2013).
J. E. Kim, S. H. Kang, Y. Moon, J. J. Chae, A. Y. Lee, J. H. Lee, K. N. Yu, D. H. Jeong, M. Choi, and M. H. Cho, Chem. Res. Toxicol., 27, No. 2, 290–303 (2014).
Y. Liu, Y. Zhao, B. Sun, and C. Chen, Acc. Chem. Res., 46, No. 3, 702–713 (2013).
J. Du, S. Wang, H. You, and X. Zhao, Environ. Toxicol. Pharmacol., 36, No. 2, 451–462 (2013).
R. Kumar and T. K. Banerjee, Clean-Soil Air Water, 41, No. 9999, 1–6 (2013).
K. K. Awasthi, P. J. John, A. Awasthi, and K. Awasthi, Micron, 44, 359–364 (2013).
R. K. Srivastava, A. B. Pant, M. P. Kashyap, V. Kumar, M. Lohani, L. Jonas, and Q. Rahman, Nanotoxicology, 5, No. 2, 195–207 (2011).
L. Whitmore and B. A. Wallace, Biopolymers, 89, No. 5, 392–400 (2008).
J. G. Lees, A. J. Miles, F. Wien, and B. A. Wallace, Bioinformatics, 22, No. 16, 1955–1962 (2006).
C. Zhang, S. Luo, and W. Chen, Talanta, 113, 142–147 (2013).
L. Canesi, R. Fabbri, G. Gallo, D. Vallotto, A. Marcomini, and G. Pojana, Aquat. Toxicol., 100, No. 2, 168–177 (2010).
M. Hossain, A. Y. Khan, and G. S. Kumar, J. Chem. Thermodyn., 47, 90–99 (2012).
Y. Fan, S. Zhang, Q. Wang, J. Li, H. Fan, and D. Shan, Appl. Spectrosc., 67, No. 6, 648–655 (2013).
J. Zhang, S. Zhuang, C. Tong, and W. Liu, J. Agric. Food Chem., 61, No. 30, 7203–7211 (2013).
Y. C. Fan, X. Y. Gong, J. Li, L. Zhang, and C. J. Sun, Spectrosc. Lett., 46, No. 4, 257–263 (2013).
Y. Z. Zhang, J. Zhang, F. F. Li, X. Xiang, A. Q. Ren, and Y. Liu, Mol. Biol. Rep., 38, No. 4, 2445–2453 (2011).
Y. Fan, S. Zhang, Q. Wang, J. Li, H. Fan, and D. Shan, Spectrochim. Acta, A, 105, 297–303 (2013).
P. D. Ross and S. Subramanian, Biochemistry, 20, No. 11, 3096–3102 (1981).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Zhurnal Prikladnoi Spektroskopii, Vol. 81, No. 5, pp. 723–728, September–October, 2014.
Rights and permissions
About this article
Cite this article
Fan, Y., Li, Y., Cai, H. et al. Fluorescence Spectrometry of the Interaction of Multi-Walled Carbon Nanotubes with Catalase. J Appl Spectrosc 81, 795–800 (2014). https://doi.org/10.1007/s10812-014-0016-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-014-0016-5