Abstract
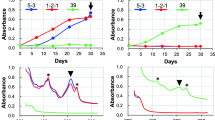
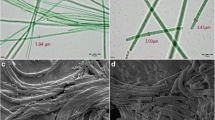
Cyanobacteria obtain their energy through photosynthesis and live embedded in a matrix of extracellular polymeric substances (EPS) containing valuable products, e.g., polysaccharides, lipids, proteins, and antimicrobials. Besides chlorophyll a and carotenoids, they have light-absorbing compounds in the form of light-harvesting complexes, the so-called phycobilisomes, consisting of different phycobiliproteins. Together they close the “green gap” whereby cyanobacteria can use light more effective than higher plants. Cultivation of cyanobacteria on a lab-scale results in small amounts of biomass for their characterization or a comprehensive screening. EPS are, for example, produced as a protection against suboptimal culture conditions. Carotenoids are essential light-harvesting pigments for photosynthesis, play a key role in photoprotective reactions, and are produced for cell wall stabilization. Essentially, the pigment composition of cyanobacteria depends on the available light spectrum, nitrogen content, and temperature. Especially the production of EPS and pigments are indicators for the cell-condition. Therefore, different EPS extraction methods were tested including the determination of inhibitory effects of extracts against Escherichia coli. Based on the best EPS extraction method, a new strategy for downstream processing (DSP) was developed to determine EPS, the pigments chlorophyll a and carotenoids, and phycobiliproteins from only one sample. As cyanobacterial model organisms Trichocoleus sociatus and Nostoc flagelliforme were used, and DSP strategy was successfully transferred to four additional cyanobacteria. The final DSP includes the following steps: (i) EPS extraction, (ii) lyophilization of biomass, (iii) extraction of phycobiliproteins, and a final (iv) chlorophyll a and carotenoid extraction. The new strategy allows a comprehensive characterization of cyanobacterial cells.






Similar content being viewed by others
Change history
22 April 2020
The original version of this article unfortunately contains mistake introduced during the publishing process. The names of the authors were interchanged in the author group. The corrected author group is shown above.
References
Bennett A, Bogorad L (1973) Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol 58:419–435
Bimakr M, Ganjloo A, Zarringhalami S, Ansarian E (2017) Ultrasound-assisted extraction of bioactive compounds from Malva sylvestris leaves and its comparison with agitated bed extraction technique. Food Sci Biotechnol 26:1481–1490
Biondi N, Tredici MR, Taton A, Wilmotte A, Hodgson DA, Losi D, Marinelli F (2008) Cyanobacteria from benthic mats of Antarctic lakes as a source of new bioactivities. J Appl Microbiol 105:105–115
Branen JK, Davidson PM (2004) Enhancement of nisin, lysozyme, and monolaurin antimicrobial activities by ethylenediaminetetraacetic acid and lactoferrin. Int J Food Microbiol 90:63–74
Braumann T, Grimme LH (1979) Single-step separation and identification of photosynthetic pigments by high-performance liquid chromatography. J Chromatogr A 1 1979:264–268
Caesar J, Tamm A, Ruckteschler N, Leifke AL, Weber B (2018) Revisiting chlorophyll extraction methods in biological soil crusts – methodology for determination of chlorophyll a and chlorophyll a + b as compared to previous methods. Biogeoscience 15:1415–1424
Caicedo NH, Heyduck-Söller B, Fischer U, Thöming J (2011) Bioproduction of antimicrobial compounds by using marine filamentous cyanobacterium cultivation. J Appl Phycol 23:811–818
Chamovitz D, Sandmann G, Hirschberg J (1993) Molecular and biochemical characterization of herbicide-resistant mutants of cyanobacteria reveals that phytoene desaturation is a rate-limiting step in carotenoid biosynthesis. J Biol Chem 268:17348–17353
Chi Z, Su CD, Lu WD (2007) A new exopolysaccharide produced by marine Cyanothece sp. 113. Bioresour Technol 98:1329–1332
Comte S, Guibaud G, Baudu M (2006) Relations between extraction protocols for activated sludge extracellular polymeric substances (EPS) and EPS complexation properties. Enzym Microb Technol 38:237–245
Dai Y-J (2013) Effect of light with different wavelengths on Nostoc flagelliforme cells in liquid culture. J Microbiol Biotechnol 23:534–538
de Rangel-Yagui CO, Danesi EDG, de Carvalho JCM, Sato S (2004) Chlorophyll production from Spirulina platensis: cultivation with urea addition by fed-batch process. Bioresour Technol 92:133–141
Dussault D, Vu KD, Vansach T, Horgen FD, Lacroix M (2016) Antimicrobial effects of marine algal extracts and cyanobacterial pure compounds against five foodborne pathogens. Food Chem 199:114–118
Flemming H-C, Neu TR, Wozniak DJ (2007) The EPS matrix: the "house of biofilm cells". J Bacteriol 189:7945–7947
Foote C S, Denny R W (1968) Chemistry of singlet oxygen. VII Quenching by beta-carotene J Am Chem Soc 90:6233–6235.
Fromme P (2008) Photosynthetic protein complexes. Wiley-VCH, Weinheim, Germany
Gantt E (1981) Phycobilisomes. Annu Rev Plant Physiol 32:327–347
Gilligan TJ, Schwarz G (1976) The self-association of adenosine-5′-triphosphate studied by circular dichroism at low ionic strengths. Biophys Chem 4:55–63
Goedheer JC (1959) Energy transfer between carotenoids and bacteriochlorophyll in chromatophores of purple bacteria. Biochim Biophys Acta 35:1–8
Goldberg SS, Cordeiro MN, Pereira SAA, Mares-Guia ML (1983) Release of lipopolysaccharide (LPS) from cell surface of Trypanosoma cruzi by EDTA. Int J Parasitol 13:11–18
Gray GW, Wilkinson SG (1965) The action of Ethylenediaminetetra-acetic acid on Pseudomonas aeruginosa. J Appl Bacteriol 28:153–164
Gross W (1995) Heterotrophic growth of two strains of the acido-thermophilic red alga Galdieria sulphuraria. Plant Cell Physiol 36:633–638
Gruszecki WI, Strzałka K (2005) Carotenoids as modulators of lipid membrane physical properties. Biochim Biophys Acta 1740:108–115
Han P-P, Shen S-G, Wang H-Y, Yao S-Y, Tan Z-L, Zhong C, Jia S-R (2017) Applying the strategy of light environment control to improve the biomass and polysaccharide production of Nostoc flagelliforme. J Appl Phycol 29:55–65
Homoelle BJ, Beck WF (1997) Solvent accessibility of the phycocyanobilin chromophore in the alpha subunit of C-phycocyanin: implications for a molecular mechanism for inertial protein-matrix solvation dynamics. Biochemistry 36:12970–12975
İlter I, Akyıl S, Demirel Z, Koç M, Conk-Dalay M, Kaymak-Ertekin F (2018) Optimization of phycocyanin extraction from Spirulina platensis using different techniques. J Food Anal 70:78–88
Kadish K M, Smith K M, Guilard R (eds) (2016) Handbook of porphyrin science (volumes 41, 42, 43, 44). With applications to chemistry, physics, materials science, engineering, biology and medicine. World scientific, Singapore
Karapanagiotis NK, Rudd T, Sterritt RM, Lester JN (1989) Extraction and characterisation of extracellular polymers in digested sewage sludge. J Chem Technol Biotechnol 44:107–120
Khazi MI, Demirel Z, Dalay MC (2018) Evaluation of growth and phycobiliprotein composition of cyanobacteria isolates cultivated in different nitrogen sources. J Appl Phycol 30:1513–1523
King RO, Forster CF (1990) Effects of sonication on activated sludge. Enzym Microb Technol 12:109–115
Koçak E, Pazir F (2018) Effect of extraction methods on bioactive compounds of plant origin. Turkish JAF Sci Tech 6:663
Kuhne S (2015) Fermentation von phototrophen Organismen zur Produktion von biotechnologischen Wertstoffen. Cuvillier Verlag, Göttingen
Kuhne S, Lakatos M, Foltz S, Muffler K, Ulber R (2013) Characterization of terrestrial cyanobacteria to increase process efficiency in low energy consuming production processes. Sustain Chem Process 1:6–6
Lakatos M, Bilger W, Büdel B (2001) Carotenoid composition of terrestrial cyanobacteria: response to natural light conditions in open rock habitats in Venezuela. Eur J Phycol 36:367–375
Lan S, Wu L, Zhang D, Hu C, Liu Y (2011) Ethanol outperforms multiple solvents in the extraction of chlorophyll-a from biological soil crusts. Soil Biol Biochem 43:857–861
Lawrenz E, Fedewa EJ, Richardson TL (2011) Extraction protocols for the quantification of phycobilins in aqueous phytoplankton extracts. J Appl Phycol 23:865–871
Leive L (1965) Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commn 21:290–296
Liang Z, Li W, Yang S, Du P (2010) Extraction and structural characteristics of extracellular polymeric substances (EPS), pellets in autotrophic nitrifying biofilm and activated sludge. Chemosphere 81:626–632
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Maccoll R (1998) Cyanobacterial phycobilisomes. J Struct Biol 124:311–334
Marvin HJ, Witholt B (1987) A highly efficient procedure for the quantitative formation of intact and viable lysozyme spheroplasts from Escherichia coli. Anal Biochem 164:320–330
Moreno J, Vargas MA, Olivares H, Rivas J, Guerrero MG (1998) Exopolysaccharide production by the cyanobacterium Anabaena sp. ATCC 33047 in batch and continuous culture. J Biotechnol 60:175–182
Nakagawa K, Ritcharoen W, Sri-Uam P, Pavasant P, Adachi S (2016) Antioxidant properties of convective-air-dried Spirulina maxima: evaluation of phycocyanin retention by a simple mathematical model of air-drying. Food Bioprod Process 100:292–302
Palmqvist K, Sltemeyer D, Baldet P, Andrews TJ, Badger MR (1995) Characterisation of inorganic carbon fluxes, carbonic anhydrase(s) and ribulose-1,5-biphosphate carboxylase-oxygenase in the green unicellular alga Coccomyxa. Planta 197:352–361
Pisciotta JM, Zou Y, Baskakov IV (2010) Light-dependent electrogenic activity of cyanobacteria. PLoS One 5:e10821
Pittera J, Partensky F, Six C (2017) Adaptive thermostability of light-harvesting complexes in marine picocyanobacteria. ISME J 11:112–124
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta - Bioenergetics 975:384–394
Pramanik A, Sundararaman M, Das S, Ghosh U, Mukherjee J (2011) Isolation and characterization of cyanobacteria possessing antimicrobial activity from the Sundarbans, the world's largest tidal mangrove forest. J Phycol 47:731–743
Rascher U, Lakatos M, Büdel B, Lüttge U (2003) Photosynthetic field capacity of cyanobacteria of a tropical inselberg of the Guiana highlands. Euo J Phycol 38:247–256
Richaud C, Zabulon G, Joder A, Thomas JC (2001) Nitrogen or sulfur starvation differentially affects phycobilisome degradation and expression of the nblA gene in Synechocystis strain PCC 6803. J Bacteriol 183:2989–2994
Rippka R, Herdman M, Waterbury JB (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Rossi F, De Philippis R (2015) Role of cyanobacterial exopolysaccharides in phototrophic biofilms and in complex microbial mats. Life 5:1218–1238
Saer RG, Blankenship RE (2017) Light harvesting in phototrophic bacteria: structure and function. Biochem J 474:2107–2131
Sato M, Murata Y, Mizusawa M, Iwahashi H, Oka S (2004) A simple and rapid dual-fluorescence viability assay for microalgae. Microbiol Cult Coll 20:53–59
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Seo YC, Choi WS, Park JH, Park JO, Jung K-H, Lee HY (2013) Stable isolation of phycocyanin from Spirulina platensis associated with high-pressure extraction process. Int J Mol Sci 14:1778–1787
Shaw E, Hill DR, Brittain N, Wright DJ, Täuber U, Marand H, Helm RF, Potts M (2003) Unusual water flux in the extracellular polysaccharide of the cyanobacterium Nostoc commune. Appl Environ Microbiol 69:5679–5684
Sheng G-P, Yu H-Q, Yu Z (2005) Extraction of extracellular polymeric substances from the photosynthetic bacterium Rhodopseudomonas acidophila. Appl Microbiol Biotechnol 67:125–130
Song W, Zhao C, Zhang D, Mu S, Pan X (2016) Different resistance to UV-B radiation of extracellular polymeric substances of two cyanobacteria from contrasting habitats. Front Microbiol 7:13
Staats N, de Winder B, Stal L, Mur L (1999) Isolation and characterization of extracellular polysaccharides from the epipelic diatoms Cylindrotheca closterium and Navicula salinarum. Eur J Phycol 34:161–169
Strieth D (2019) Produktive phototrophe Biofilme in Aerosolreaktoren. 1 Verlag Dr. Hut (Verfahrenstechnik), Munich
Strieth D, Schwing J, Kuhne S, Lakatos M, Muffler K, Ulber R (2017) A semi-continuous process based on an ePBR for the production of EPS using Trichocoleus sociatus. J Biotechnol 256:6–12
Vaara M (1981) Increased outer membrane resistance to ethylenediaminetetraacetate and cations in novel lipid a mutants. J Bacteriol 148:426–434
Wang B, Yang L, Zhang Y, Chen S, Gao X, Wan C (2019) Investigation of the dynamical expression of Nostoc flagelliforme proteome in response to rehydration. J Proteome 192:160–168
Weber B, Wessels DCJ, Deutschewitz K, Dojani S, Reichenberger H, Büdel B (2013) Ecological characterization of soil-inhabiting and hypolithic soil crusts within the Knersvlakte. S Afr Ecol Proc 2:231–213
Yi Z-W, Huang H, Kuang T-Y, Sui S-F (2005) Three-dimensional architecture of phycobilisomes from Nostoc flagelliforme revealed by single particle electron microscopy. FEBS Lett 579:3569–3573
Yu H, Liu R (2013) Effect of UV-B radiation on the synthesis of UV-absorbing compounds in a terrestrial cyanobacterium, Nostoc flagelliforme. J Appl Phycol 25:1441–1446
Acknowledgments
We would like to thank Prof. Dr. Burkhard Büdel (University of Kaiserslautern, Department of Plant Ecology and Systematics, Germany) for his expertise, his stock cultures, and his assistance concerning cyanobacteria.
Funding
The research was supported by the Carl-Zeiss-Stiftung, TU-Nachwuchsring research funding, the German Research Foundation (DFG; Project number: UL 170/16–1; MU 2985/3–1), Ministry of Education of Rhineland-Palatinate (bm.rlp) (iProcess intelligent process development – from modeling to product) and the Federal Ministry of Education and Research (BMBF) (Project number: 031B0068D).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Strieth, D., Stiefelmaier, J., Wrabl, B. et al. A new strategy for a combined isolation of EPS and pigments from cyanobacteria. J Appl Phycol 32, 1729–1740 (2020). https://doi.org/10.1007/s10811-020-02063-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02063-x