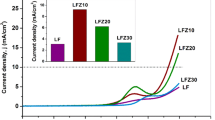
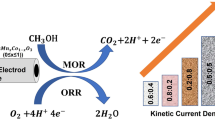
Abstract
Four transition metals, namely cobalt, iron, manganese, and nickel, were separately combined with lanthanum metal to synthesize LaMO3 (M = Co, Fe, Mn, and Ni) perovskites using a sol–gel method. Electrodes for zinc–air rechargeable batteries were prepared with these perovskites and evaluated in terms of their morphology, crystal structure, electric conductivity, surface area, and particle size distribution. The electrochemical properties of the perovskites were characterized as catalysts of bifunctional electrodes for Hgen reduction reaction and oxygen evolution reaction in alkaline solution. Additionally, partially substituted LaNi x (M1, M2, or M3)1−x O3 (x = 0.25 or 0.5, and M1, 2, 3 = Co, Fe, Mn) perovskites were synthesized to evaluate the effect of partial substitution of a metal in the Ni site, and improved physical and electrochemical properties were obtained.













Similar content being viewed by others
References
Hilder M, Winther-Jensen B, Clark NB (2012) The effect of binder and electrolyte on the performance of thin zinc–air battery. Electrochim Acta 69:308–314
Wang X, Sebastian PJ, Smit MA, Yang H, Gamboa SA (2003) Studies on the oxygen reduction catalyst for zinc–air battery electrode. J Power Sources 124:278–284
Li X, Qu W, Zhang J, Wang H (2010) Electrocatalytic activities of perovskite toward oxygen reduction reaction in concentrated alkaline electrolytes. ESC Trans 28:45–56
Lee SH, Jeong Y, Lim S, Lee EA, Yi CW, Kim K (2010) The stable rechargeability of secondary Zn–air batteries: is it possible to recharge a Zn–air battery? J Korean Electrochem Soc 13:45–49
Wang T, Kaempgen M, Nopphawan P, Wee G, Mhaisalkar S, Srinivasan M (2010) Silver nanoparticle-decorated carbon nanotubes as bifunctional gas-diffusion electrodes for zinc–air batteries. J Power Sources 195:4350–4355
Lee JS, Kim ST, Cao R, Choi NS, Liu M, Lee KT, Cho J (2011) Metal-air batteries with high energy density: Li–air versus Zn–air. Adv Energy Mater 1:34–50
Shim J, Park YS, Lee HK, Park SG, Lee JS (1996) Oxygen reduction reaction of La1-xCaxCoO3 of gas diffusion electrode in alkaline fuel cell. J Korean Ind Eng Chem 7:992–998
Jorissen L (2006) Bifunctional oxygen/air electrodes. J Power Sources 155:23–32
Chen Z, Choi JY, Wang H, Li H, Chen Z (2011) Highly durable and active non-precious air cathode catalyst for zinc air battery. J Power Sources 196:3673–3677
Chen Z, Yu A, Higgins D, Li H, Wang H, Chen Z (2012) Highly active and durable core-corona structured bifunctional catalyst for rechargeable metal-air battery application. Nano Lett 12:1946–1952
Zhou W, Sunarso J (2013) Enhancing Bi-functional electrocatalytic activity of perovskite by temperature shock: a case study of LaNiO3−δ. J Phys Chem Lett 4:2982–2988
Hardin WG, Slanac DA, Wang X, Dai S, Johnston KP, Stevenson KJ (2013) Highly active, nonprecious metal perovskite electrocatalysts for bifunctional metal-air battery electrodes. J Phys Chem Lett 4:1254–1259
Zhang H, Li N, Li K, Xue D (2007) Structural stability and formability of ABO3-type perovskite compounds. Acta Crystallogr Sect B 63:812–818
Zhuang S, Liu S, Huang C, Tu F, Zhang J, Li Y (2012) Electrocatalytic activity of nanoporous perovskite La1-xCaxCoO3 towards hydrogen peroxide reduction in alkaline medium. Int J Electrochem Sci 7:338–344
Neburchilov V, Wang H, Martin JJ, Qu W (2010) A review on air cathodes for zinc–air fuel cells. J Power Sources 195:1271–1291
Suntivich J, Gasteiger HA, Yabuuchi N, Nakanishi H, Goodenough JB, Shao-Horn Y (2011) Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal-air batteries. Nat Chem 3:546–550
Pechini MP (1967) Method of preparing lead and alkaline earth titanates and niobates and coating method using the same to form a capacitor. US Patent No. 3.330.697
Popa M, Kakihana M (2002) Synthesis of lanthanum cobaltite (LaCoO3) by the polymerizable complex route. Solid State Ion 151:251–257
Kuo JH, Anderson HU, Sparlin DM (1990) Oxidation-reduction behavior of undoped and Sr-doped LaMnO3: defect structure, electrical conductivity, and thermoelectric power. J Solid State Chem 87:55–63
Worayingyong A, Kangvansura P, Ausadasuk S, Praserthdam P (2008) The effect of preparation: Pechini and Schiff base methods, on adsorbed oxygen of LaCoO3 perovskite oxidation catalysts. Colloids Surf A 315:217–225
Popa M, Frantti J, Kakihana M (2002) Characterization of LaMeO3 (Me: Mn Co, Fe) perovskite powders obtained by polymerizable complex method. Solid State Ion 154–155:135–141
Fernandes JDG, Melo DMA, Zinner LB, Salustiano CM, Silva ZR, Martinelli AE, Cerqueira M, Alves Júnior C, Longo E, Bernardi MIB (2002) Low-temperature synthesis of single-phase crystalline LaNiO3 perovskite via Pechini method. Mater Lett 53:122–125
Ahn S, Kim K, Kim H, Nam S, Eom S (2010) Synthesis and electrochemical performance of La0.7Sr0.3Co1−xFexO3 catalysts for zinc air secondary batteries. Phys Scr T139:014014
Matsushima H, Majima W, Fukunaka Y (2013) Three-phase interfacial phenomena in alkaline unitized regenerative fuel cell. Electrochim Acta 114:509–513
Matsuno Y, Suzawa K, Tsutsumi A, Yoshida K (1996) Characteristics of three-phase fluidized-bed electrodes for an alkaline fuel cell cathode. Int J Hydrog Energy 21:195–199
Singh NK, Lal B, Singh RN (2002) Electrocatalytic properties of perovskite-type La1−xSrxMnO3 obtained by a novel sol–gel route for O2 evolution in KOH solutions. Int J Hydrogen Energy 27:885–893
Singh RN, Tiwari SK, Singh SP, Jain AN, Singh NK (1997) Electrocatalytic activity of high specific surface area perovskite-type LaNiO3 via sol–gel route for electrolytic oxygen evolution in alkaline solution. Int J Hydrog Energy 22:557–562
Lal B, Raghunandan MK, Gupta M, Singh RN (2005) Electrocatalytic properties of perovskite-type La1-xSrxCoO3 (0≤×≤4) obtained by a novel stearic acid sol–gel method for electrocatalysis of O2 evolution in KOH solutions. Int J Hydrog Energy 30:723–729
Bursell M, Pirjamali M, Kiros Y (2002) La0.6Ca0.4CoO3, La0.1Ca0.9MnO3 and LaNiO3 as bifunctional oxygen electrodes. Electrochim Acta 47:1651–1660
Suresh K, Panchapagesan TS, Patil KC (1999) Synthesis and properties of La1−xSrxFeO3. Solid State Ion 126:299–305
Sarma DD, Shanthi N, Barman SR (1995) Band Theory for Ground-State Properties and Excitation Spectra of Perovskite LaMO3 (M = Mn, Fe Co, Ni). Phys Rev Lett 75:1126–1129
Hyodo T, Shimizu Y, Miura N, Yamazoe N (1994) Investigation of materials for gas diffusion-type oxygen cathode aiming at electric power-saving brine electrolysis. Denki Kagaku 62:158–164
Acknowledgments
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (MEST) (NRF-2012-M1A2A2-029538).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lopez, K., Park, G., Sun, HJ. et al. Electrochemical characterizations of LaMO3 (M = Co, Mn, Fe, and Ni) and partially substituted LaNi x M1−x O3 (x = 0.25 or 0.5) for oxygen reduction and evolution in alkaline solution. J Appl Electrochem 45, 313–323 (2015). https://doi.org/10.1007/s10800-015-0798-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-015-0798-z