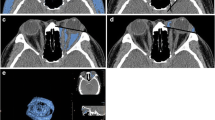
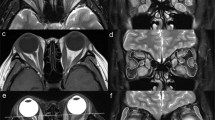
Abstract
Purpose
This study aimed to explore the diagnostic value of whole-orbit-based multiparametric assessment on Dixon MRI for the evaluation of the thyroid eye disease (TED) activity.
Methods
The retrospective study enrolled patients diagnosed as TED and obtained their axial and coronal Dixon MRI scans. Multiparameters were assessed, including water fraction (WF), fat fraction (FF) of extraocular muscles (EOMs), orbital fat (OF), and lacrimal gland (LG). The thickness of OF and herniation of LG were also measured. Univariable and multivariable logistic regression was applied to construct prediction models based on single or multiple structures. Receiver operating characteristic (ROC) curve analysis was also implemented.
Results
Univariable logistic analysis revealed significant differences in water fraction (WF) of the superior rectus (P = 0.018), fat fraction (FF) of the medial rectus (P = 0.029), WF of OF (P = 0.004), and herniation of LG (P = 0.012) between the active and inactive TED phases. Multivariable logistic analysis and corresponding receiver operating characteristic curve (ROC) analysis of each structure attained the area under the curve (AUC) values of 0.774, 0.771, and 0.729 for EOMs, OF, and LG, respectively, while the combination of the four imaging parameters generated a final AUC of 0.909.
Conclusions
Dixon MRI may be used for fine multiparametric assessment of multiple orbital structures. The whole-orbit-based model improves the diagnostic performance of TED activity evaluation.



Similar content being viewed by others
Data availability
Available on request.
References
Bahn RS (2010) Graves’ ophthalmopathy. N Engl J Med 362(8):726–738. https://doi.org/10.1056/NEJMra0905750
Douglas RS et al (2020) Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med 382(4):341–352. https://doi.org/10.1056/NEJMoa1910434
Bartalena L, Tanda ML (2022) Current concepts regarding Graves’ orbitopathy. J Intern Med 292(5):692–716. https://doi.org/10.1111/joim.13524
Bartalena L et al (2021) The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol 185(4):G43–G67. https://doi.org/10.1530/EJE-21-0479
Li Y et al (2024) Use of extreme gradient boosting, light gradient boosting machine, and deep neural networks to evaluate the activity stage of extraocular muscles in thyroid-associated ophthalmopathy. Graefes Arch Clin Exp Ophthalmol 262(1):203–210. https://doi.org/10.1007/s00417-023-06256-1
Mawn LA et al (2018) Soft tissue metrics in thyroid eye disease: an international thyroid eye disease society reliability study. Ophthalmic Plast Reconstr Surg 34(6):544–546. https://doi.org/10.1097/IOP.0000000000001080
Bartalena L et al (2008) Consensus statement of the European Group on Graves’ orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol 158(3):273–285. https://doi.org/10.1530/EJE-07-0666
Tachibana S et al (2010) Orbital magnetic resonance imaging combined with clinical activity score can improve the sensitivity of detection of disease activity and prediction of response to immunosuppressive therapy for Graves’ ophthalmopathy. Endocr J 57(10):853–861. https://doi.org/10.1507/endocrj.k10e-156
Das T et al (2019) T2-relaxation mapping and fat fraction assessment to objectively quantify clinical activity in thyroid eye disease: an initial feasibility study. Eye (Lond) 33(2):235–243. https://doi.org/10.1038/s41433-018-0304-z
Lecler A (2021) Expanding diagnostic tools for dysthyroid optic neuropathy: how quantitative MRI can be used to visualize and measure orbital inflammation. Eur Radiol 31(10):7417–7418. https://doi.org/10.1007/s00330-021-08208-x
Zhang H et al (2023) Predictive markers for anti-inflammatory treatment response in thyroid eye disease. Front Endocrinol (Lausanne) 14:1292519. https://doi.org/10.3389/fendo.2023.1292519
Liu X et al (2021) Application of magnetic resonance imaging in the evaluation of disease activity in Graves’ ophthalmopathy. Endocr Pract 27(3):198–205. https://doi.org/10.1016/j.eprac.2020.09.008
Ge Q et al (2021) Quantitative evaluation of activity of thyroid-associated ophthalmopathy using short-tau inversion recovery (STIR) sequence. BMC Endocr Disord 21(1):226. https://doi.org/10.1186/s12902-021-00895-3
Lingam RK, Mundada P, Lee V (2018) Novel use of non-echo-planar diffusion weighted MRI in monitoring disease activity and treatment response in active Grave’s orbitopathy: an initial observational cohort study. Orbit 37(5):325–330. https://doi.org/10.1080/01676830.2017.1423343
Higashiyama T, Iwasa M, Ohji M (2017) Quantitative analysis of inflammation in orbital fat of thyroid-associated ophthalmopathy using MRI signal intensity. Sci Rep 7(1):16874. https://doi.org/10.1038/s41598-017-17257-6
Ollitrault A et al (2021) Dixon-T2WI magnetic resonance imaging at 3 tesla outperforms conventional imaging for thyroid eye disease. Eur Radiol 31(7):5198–5205. https://doi.org/10.1007/s00330-020-07540-y
Wong NTY et al (2023) Magnetic resonance imaging parameters on lacrimal gland in thyroid eye disease: a systematic review and meta-analysis. BMC Ophthalmol 23(1):347. https://doi.org/10.1186/s12886-023-03008-x
Zhang H et al (2023) Application of quantitative MRI in thyroid eye disease: imaging techniques and clinical practices. J Magn Reson Imaging. https://doi.org/10.1002/jmri.29114
Hu H et al (2020) Predicting the response to glucocorticoid therapy in thyroid-associated ophthalmopathy: mobilizing structural MRI-based quantitative measurements of orbital tissues. Endocrine 70(2):372–379. https://doi.org/10.1007/s12020-020-02367-5
Cheng J et al (2023) Evaluation of activity of Graves’ orbitopathy with multiparameter orbital magnetic resonance imaging (MRI). Quant Imaging Med Surg 13(5):3040–3049. https://doi.org/10.21037/qims-22-814
Zhang H et al (2024) Whole-orbit radiomics: machine learning-based multi- and fused- region radiomics signatures for intravenous glucocorticoid response prediction in thyroid eye disease. J Transl Med 22(1):56. https://doi.org/10.1186/s12967-023-04792-2
Bartalena L (2013) Diagnosis and management of Graves disease: a global overview. Nat Rev Endocrinol 9(12):724–734. https://doi.org/10.1038/nrendo.2013.193
Song C et al (2023) Extraocular muscle volume index at the orbital apex with optic neuritis: a combined parameter for diagnosis of dysthyroid optic neuropathy. Eur Radiol 33(12):9203–9212. https://doi.org/10.1007/s00330-023-09848-x
Xu L et al (2017) Thickness of extraocular muscle and orbital fat in MRI predicts response to glucocorticoid therapy in Graves’ ophthalmopathy. Int J Endocrinol 2017:3196059. https://doi.org/10.1155/2017/3196059
Gagliardo C et al (2020) Lacrimal gland herniation in Graves ophthalmopathy: a simple and useful MRI biomarker of disease activity. Eur Radiol 30(4):2138–2141. https://doi.org/10.1007/s00330-019-06570-5
Wu D et al (2021) Utility of multi-parametric quantitative magnetic resonance imaging of the lacrimal gland for diagnosing and staging Graves’ ophthalmopathy. Eur J Radiol 141:109815. https://doi.org/10.1016/j.ejrad.2021.109815
Chen L et al (2021) Usefulness of two-point Dixon T2-weighted imaging in thyroid-associated ophthalmopathy: comparison with conventional fat saturation imaging in fat suppression quality and staging performance. Br J Radiol 94(1118):20200884. https://doi.org/10.1259/bjr.20200884
Armstrong RA (2013) Statistical guidelines for the analysis of data obtained from one or both eyes. Ophthalmic Physiol Opt 33(1):7–14. https://doi.org/10.1111/opo.12009
Ma Z et al (2019) Improvement of the MRI and clinical features of Asian Graves’ ophthalmopathy by radiation therapy with steroids. Jpn J Radiol 37(8):612–618. https://doi.org/10.1007/s11604-019-00846-y
Das T et al (2019) T2-relaxation mapping and fat fraction assessment to objectively quantify clinical activity in thyroid eye disease: an initial feasibility study. Eye 33(2):235–243. https://doi.org/10.1038/s41433-018-0304-z
Chaobin ZYHXZZLCZ (2021) Clinical significance of Dixon quantificatioin in evaluation of thyroid⁃related ophthalmopathy. Chin J Biomed Eng. https://doi.org/10.3760/cma.j.cn115668⁃20210601⁃00112
Lee BW et al (2018) Transcriptome analysis of orbital adipose tissue in active thyroid eye disease using next generation RNA sequencing technology. Open Ophthalmol J 12:41–52. https://doi.org/10.2174/1874364101812010041
Garau LM et al (2018) Extraocular muscle sampled volume in Graves’ orbitopathy using 3-T fast spin-echo MRI with iterative decomposition of water and fat sequences. Acta Radiol Open 7(6):2058460118780892. https://doi.org/10.1177/2058460118780892
Nagy EV et al (2000) Graves’ ophthalmopathy: eye muscle involvement in patients with diplopia. Eur J Endocrinol 142(6):591–597. https://doi.org/10.1530/eje.0.1420591
Imbrasienė D, Jankauskienė J, Stanislovaitienė D (2010) Ultrasonic measurement of ocular rectus muscle thickness in patients with Graves’ ophthalmopathy. Medicina (Kaunas) 46(7):472–476
Keene KR et al (2021) The feasibility of quantitative MRI of extra-ocular muscles in myasthenia gravis and Graves’ orbitopathy. NMR Biomed 34(1):4407. https://doi.org/10.1002/nbm.4407
Kaichi Y et al (2019) Thyroid-associated orbitopathy: quantitative evaluation of the orbital fat volume and edema using IDEAL-FSE. Eur J Radiol Open 6:182–186. https://doi.org/10.1016/j.ejro.2019.05.003
Nedeljkovic Beleslin B et al (2014) Efficacy and safety of combined parenteral and oral steroid therapy in Graves’ orbitopathy. Hormones (Athens) 13(2):222–228. https://doi.org/10.1007/BF03401336
van Vucht N et al (2019) The Dixon technique for MRI of the bone marrow. Skeletal Radiol 48(12):1861–1874. https://doi.org/10.1007/s00256-019-03271-4
Ma J (2004) Breath-hold water and fat imaging using a dual-echo two-point Dixon technique with an efficient and robust phase-correction algorithm. Magn Reson Med 52(2):415–419. https://doi.org/10.1002/mrm.20146
Reeder SB et al (2004) Multicoil Dixon chemical species separation with an iterative least-squares estimation method. Magn Reson Med 51(1):35–45. https://doi.org/10.1002/mrm.10675
Huang K et al (2023) Image quality and evaluation ability of magnetic resonance imaging techniques for thyroid-associated ophthalmopathy: Dixon fat-suppression technique vs. spectral attenuated inversion recovery. Front Med (Lausanne) 10:1154828. https://doi.org/10.3389/fmed.2023.1154828
Gaddikeri S et al (2018) Optimal fat suppression in head and neck mri: comparison of multipoint Dixon with 2 different fat-suppression techniques, spectral presaturation and inversion recovery, and STIR. AJNR Am J Neuroradiol 39(2):362–368. https://doi.org/10.3174/ajnr.A5483
Kijowski R et al (2009) Improved fat suppression using multipeak reconstruction for IDEAL chemical shift fat-water separation: application with fast spin echo imaging. J Magn Reson Imaging 29(2):436–442. https://doi.org/10.1002/jmri.21664
Dixon WT (1984) Simple proton spectroscopic imaging. Radiology 153(1):189–194. https://doi.org/10.1148/radiology.153.1.6089263
Ma J (2008) Dixon techniques for water and fat imaging. J Magn Reson Imaging 28(3):543–558. https://doi.org/10.1002/jmri.21492
Acknowledgements
We thank Ms. Qingwen Tang, Ms. Qi Zheng, and Mr. Zhenhua Zhang for their assistance in data collation. We also thank Shanghai Medoo Tech Company for their technical suggestions
Funding
This study was funded by the National Natural Science Foundation of China (82071003 and 82271122), the Science and Technology Commission of Shanghai (20DZ22708), the Shanghai Key Clinical Specialty, Shanghai Eye Disease Research Center (2022ZZ01003), the Clinical Acceleration Program of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (JYLJ202202), Clinical Research Program of 9th People’s Hospital, Shanghai Jiao Tong University School of Medicine (JYLJ202120).
Author information
Authors and Affiliations
Contributions
Conceptualization: Huifang Z and Xuefei S. Formal analysis: Duojin X., Hui W. and Haiyang Z. Resources: Yinwei L. and Jing S. Data curation: Mengda J. and Yan.T Writing—original draft preparation: Duojin X . Writing—review and editing: Haiyang Z and Duojin X. Supervision: Xuefei S. Project administration: Huifang Z and Xuefei S. Funding acquisition Huifang Z. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Institutional Review Board (SH9H-2021-T246-2), and the informed consent requirement was waived. It was in compliance with the tenets of the Declaration of Helsinki for clinical research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xia, D., Zhang, H., Wang, H. et al. Whole-orbit-based multiparametric assessment of disease activity of thyroid eye disease on Dixon MRI. Int Ophthalmol 44, 213 (2024). https://doi.org/10.1007/s10792-024-03138-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10792-024-03138-1