Abstract
Purpose
To compare results of treatment with bevacizumab and ranibizumab injections in myopic choroidal neovascularization (mCNV).
Methods
Retrospective, observational case series. Participants: patients with mCNV treated with bevacizumab or ranibizumab injections. Best corrected visual acuity (BCVA) and central retinal thickness (CRT) on optical coherence tomography (OCT) scans were collected at baseline, after 3, 6, 12, 24 months and the last visit. Main outcome measures: mean change in BCVA and CRT.
Results
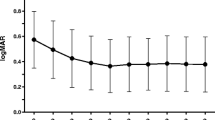
We included 85 eyes treated with bevacizumab and 125 eyes treated with ranibizumab. There was no difference between the groups regarding BCVA and CRT change. CNV recurrence occurred at the mean time of 66.1 ± 3.7 and 57.3 ± 6.4 months in the bevacizumab- and ranibizumab-treated eyes, respectively (p = 0.006). During the first year 6.9% eyes in the bevacizumab group vs. 27.5% in the ranibizumab group had CNV recurrence (p = 0.001). Risk factors for recurrence of CNV were baseline CNV area (aHR 1.20, 95%CI 1.0–1.32, p = 0.04), subfoveal CNV (aHR 2.13, 95% CI 1.16–3.93, p = 0.01) and ranibizumab treatment (aHR 2.31, 95% CI 1.16–3.93, p = 0.008).
Conclusion
Eyes treated with bevacizumab and ranibizumab can achieve similar anatomical and functional improvement. CNV recurrence may occur earlier and more frequently during the first year in eyes treated with ranibizumab.

Similar content being viewed by others
References
Neelam K, Cheung CMG, Ohno-Matsui K, Lai TYY, Wong TY (2012) Choroidal neovascularization in pathological myopia. Prog Retin Eye Res 31(5):495–525
Cohen SY, Laroche A, Leguen Y, Soubrane G, Coscas GJ (1996) Etiology of choroidal neovascularization in young patients. Ophthalmology 103(8):1241–1244
Ohno-Matsui K, Ikuno Y, Lai TYY, Gemmy Cheung CM (2018) Diagnosis and treatment guideline for myopic choroidal neovascularization due to pathologic myopia. Prog Retin Eye Res 63:92–106
Bruyère E, Miere A, Cohen SY et al (2017) Neovascularization secondary to high myopia imaged by optical coherence tomography angiography. Retina 37(11):2095–2101
Wolf S, Balciuniene VJ, Laganovska G et al (2014) RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology 121(3):682–92.e2
Ikuno Y, Ohno-Matsui K, Wong TY et al (2015) Intravitreal aflibercept injection in patients with myopic choroidal neovascularization: the MYRROR study. Ophthalmology 122(6):1220–1227
Tomkins-Netzer O, Talat L, Bar A et al (2014) Long-term clinical outcome and causes of vision loss in patients with uveitis. Ophthalmology 121(12):2387–2392
WHO (2017) Change the definition of blindness. Available at: who.int/blindness/ [Accessed May 17, 2017]
Ohno-Matsui K, Lai TYY, Lai C-C, Cheung CMG (2016) Updates of pathologic myopia. Prog Retin Eye Res 52:156–187
Macular Photocoagulation Study Group (1991) Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol 109(9):1220–1231
Ohno-Matsui K, Kawasaki R, Jonas JB et al (2015) International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol 159(5):877–83.e7
Gharbiya M, Giustolisi R, Allievi F et al (2010) Choroidal neovascularization in pathologic myopia: intravitreal ranibizumab versus bevacizumab–a randomized controlled trial. Am J Ophthalmol 149(3):458–64.e1
Pece A, Milani P, Monteleone C et al (2015) A randomized trial of intravitreal bevacizumab vs ranibizumab for myopic CNV. Graefe’s Arch Clin Exp Ophthalmol = Albr von Graefes Arch fur Klin und Exp Ophthalmol 253(11):1867–1872
Cha DM, Kim TW, Heo JW et al (2014) Comparison of 1-year therapeutic effect of ranibizumab and bevacizumab for myopic choroidal neovascularization: a retrospective, multicenter, comparative study. BMC Ophthalmol 14:69
Iacono P, Parodi MB, Papayannis A et al (2012) Intravitreal ranibizumab versus bevacizumab for treatment of myopic choroidal neovascularization. Retina 32(8):1539–1546
Yang HS, Kim J-G, Kim JT, Joe SG (2013) Prognostic factors of eyes with naïve subfoveal myopic choroidal neovascularization after intravitreal bevacizumab. Am J Ophthalmol 156(6):1201-1210.e2
Lai TYY, Luk FOJ, Lee GKY, Lam DSC (2012) Long-term outcome of intravitreal anti-vascular endothelial growth factor therapy with bevacizumab or ranibizumab as primary treatment for subfoveal myopic choroidal neovascularization. Eye (Lond) 26(7):1004–1011
Ng DS-C, Kwok AKH, Tong JM-K, Chan CW-N, Li WW-T (2015) Factors influencing need for retreatment and long-term visual outcome after intravitreal bevacizumab for myopic choroidal neovascularization. Retina 35(12):2457–2468
Gharbiya M, Cruciani F, Parisi F, Cuozzo G, Altimari S, Abdolrahimzadeh S (2012) Long-term results of intravitreal bevacizumab for choroidal neovascularisation in pathological myopia. Br J Ophthalmol 96(8):1068–1072
Ceklic L, Wolf-Schnurrbusch U, Gekkieva M, Wolf S (2014) Visual acuity outcome in RADIANCE study patients with dome-shaped macular features. Ophthalmology 121(11):2288–2289
Lee JH, Lee SC, Choi S, Koh HJ, Kim SS, Lee CS (2017) Two-year outcomes of intravitreal bevacizumab for choroidal neovascularization associated with a dome-shaped macula in pathologic myopia. Eye (Lond) 31(3):507–508
Farinha CL, Baltar AS, Nunes SG et al (2014) Progression of myopic maculopathy after treatment of choroidal neovascularization. Ophthalmol J Int d’ophtalmologie Int J Ophthalmol Zeitschrift fur Augenheilkd 231(4):211–220
Hampton GR, Kohen D, Bird AC (1983) Visual prognosis of disciform degeneration in myopia. Ophthalmology 90(8):923–926
Hayashi K, Shimada N, Moriyama M, Hayashi W, Tokoro T, Ohno-Matsui K (2012) Two-year outcomes of intravitreal bevacizumab for choroidal neovascularization in Japanese patients with pathologic myopia. Retina 32(4):687–695
Ikuno Y, Sayanagi K, Soga K et al (2009) Intravitreal bevacizumab for choroidal neovascularization attributable to pathological myopia: one-year results. Am J Ophthalmol 147(1):94-100.e1
Ceklic L, Munk MR, Wolf-Schnurrbusch U, Gekkieva M, Wolf S (2017) Visual acuity outcomes of ranibizumab treatment in pathologic myopic eyes with macular retinoschisis and choroidal neovascularization. Retina 37(4):687–693
Peiretti E, Vinci M, Fossarello M (2012) Intravitreal bevacizumab as a treatment for choroidal neovascularisation secondary to myopia: 4-year study results. Can J Ophthalmol 47(1):28–33
Goto S, Sayanagi K, Ikuno Y, Jo Y, Gomi F, Nishida K (2015) Comparison of visual prognoses between natural course of simple hemorrhage and choroidal neovascularization treated with intravitreal bevacizumab in highly myopic eyes: a 1-year follow-up. Retina 35(3):429–434
Wakabayashi T, Ikuno Y, Gomi F (2011) Different dosing of intravitreal bevacizumab for choroidal neovascularization because of pathologic myopia. Retina 31(5):880–886
Monés JM, Amselem L, Serrano A, Garcia M, Hijano M (2009) Intravitreal ranibizumab for choroidal neovascularization secondary to pathologic myopia: 12-month results. Eye (Lond) 23(6):1275–1280
Wu T-T, Kung Y-H (2012) The 12-month outcome of three consecutive monthly intravitreal injections of ranibizumab for myopic choroidal neovascularization. J Ocul Pharmacol Ther Off J Assoc Ocul Pharmacol Ther 28(2):129–133
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Malgorzata Woronkowicz and Sophia Zagora. The first draft of the manuscript was written by Malgorzata Woronkowicz and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Financial interests: Robin Hamilton received payment for lectures including service on speaker bureaus from Novartis, Bayer and Allergan. Oren Tomkins-Netzer received payment for consultancy from Abbvie inc. Non-financial interests: none
Conflict of interest
Robin Hamilton received payment for lectures including service on speaker bureaus from Novartis, Bayer and Allergan. Oren Tomkins–Netzer received payment for consultancy from Abbvie inc.
Ethical approval
The study received Institutional Review Board approval and was conducted in accordance with the tenets of the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Woronkowicz, M., Hamilton, R., Lightman, S. et al. Comparison of anatomical and functional outcomes of treating myopic choroidal neovascularization with bevacizumab or ranibizumab. Int Ophthalmol 43, 3499–3507 (2023). https://doi.org/10.1007/s10792-023-02755-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-023-02755-6