Abstract
Purpose
The proliferation and angiogenesis of human retinal endothelial cells (HRECs) are critical for the pathophysiology of diabetic retinopathy (DR). C-terminal binding protein 2 (CtBP2) has multiple biologic functions, but its effect on HRECs under high-glucose (HG) conditions is unclear.
Methods
The cell viability, angiogenesis, cellular adhesion and CtBP2 expression levels of HRECs were measured following treatment with different concentrations of glucose. Small interfering CtBP2-targeting RNA, wide-type and function mutant plasmid of CtBP2 were constructed and then were transfected into HRECs to evaluate the effects of CtBP2 on cell functions of HRECs.
Results
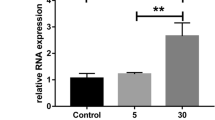
The expression of CtBP2 in HRECs was increased after HG treatment. HG treatment significantly increased cell proliferation, angiogenesis, and decreased relative gene expressions in gap junctions, tight junctions and adherens junctions. After CtBP2 was inhibited via siRNA, the changes induced by HG were partially restored. Conversely, only wild-type CtBP2 could increase cell proliferation and angiogenesis under HG condition. Mechanistically, we also found that CtBP2 exerted its functions to effect HG-induced changes via Akt signaling pathway.
Conclusion
This study implicates that CtBP2 promotes HG-induced cell proliferation, angiogenesis and cellular adhesion, and CtBP2 might be a potential target in the prevention of DR.




Similar content being viewed by others
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Abbreviations
- DR:
-
Diabetic retinopathy
- HRECs:
-
Human retinal endothelial cells
- CtBP2:
-
C-terminal binding protein 2
- GJs:
-
Gap junctions
- TJs:
-
Tight junctions
- AJs:
-
Adherens junctions
- HG:
-
High glucose
- LG:
-
Low glucose
- MAPK:
-
Mitogen-activated protein kinase
- PI3K:
-
Phosphatidylinositol-3-kinase
- VEGF:
-
Vascular endothelial growth factor
- qRT-PCR:
-
Real-time quantitative reverse transcription PCR
- ZEB1:
-
Zinc finger E-box binding homeobox 1
- TNF-α:
-
Tumor necrosis factor alpha
- ZO-1:
-
Zonula occludens-1
- NADH:
-
Nicotinamide adenine dinucleotide
- PTEN:
-
Phosphatase and tensin homolog
References
Shaw JE, Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87:4–14. https://doi.org/10.1016/j.diabres.2009.10.007
Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME (2008) Vascular endothelial growth factor in eye disease. Prog Retin Eye Res 27:331–371. https://doi.org/10.1016/j.preteyeres.2008.05.001
Behl T, Kotwani A (2015) Exploring the various aspects of the pathological role of vascular endothelial growth factor (VEGF) in diabetic retinopathy. Pharmacol Res 99:137–148. https://doi.org/10.1016/j.phrs.2015.05.013
Huang H, He J, Johnson D, Wei Y, Liu Y, Wang S, Lutty GA, Duh EJ, Semba RD (2015) Deletion of placental growth factor prevents diabetic retinopathy and is associated with Akt activation and HIF1alpha-VEGF pathway inhibition. Diabetes 64:200–212. https://doi.org/10.2337/db14-0016
Zhang Y, Jiang X, Qin X, Ye D, Yi Z, Liu M, Bai O, Liu W, Xie X, Wang Z, Fang J, Chen Y (2010) RKTG inhibits angiogenesis by suppressing MAPK-mediated autocrine VEGF signaling and is downregulated in clear-cell renal cell carcinoma. Oncogene 29:5404–5415. https://doi.org/10.1038/onc.2010.270
Joussen AM, Poulaki V, Tsujikawa A, Qin W, Qaum T, Xu Q, Moromizato Y, Bursell SE, Wiegand SJ, Rudge J, Ioffe E, Yancopoulos GD, Adamis AP (2002) Suppression of diabetic retinopathy with angiopoietin-1. Am J Pathol 160:1683–1693. https://doi.org/10.1016/S0002-9440(10)61115-7
Roy S, Kim D, Lim R (2017) Cell-cell communication in diabetic retinopathy. Vision Res 139:115–122. https://doi.org/10.1016/j.visres.2017.04.014
Ruggiero D, Lecomte M, Michoud E, Lagarde M, Wiernsperger N (1997) Involvement of cell-cell interactions in the pathogenesis of diabetic retinopathy. Diabetes Metab 23:30–42
Sawada N, Murata M, Kikuchi K, Osanai M, Tobioka H, Kojima T, Chiba H (2003) Tight junctions and human diseases. Med Electron Microsc 36:147–156. https://doi.org/10.1007/s00795-003-0219-y
Dagli ML, Hernandez-Blazquez FJ (2007) Roles of gap junctions and connexins in non-neoplastic pathological processes in which cell proliferation is involved. J Membr Biol 218:79–91. https://doi.org/10.1007/s00232-007-9045-9
Aveleira CA, Lin CM, Abcouwer SF, Ambrosio AF, Antonetti DA (2010) TNF-alpha signals through PKCzeta/NF-kappaB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes 59:2872–2882. https://doi.org/10.2337/db09-1606
Platania CBM, Lazzara F, Fidilio A, Fresta CG, Conti F, Giurdanella G, Leggio GM, Salomone S, Drago F, Bucolo C (2019) Blood-retinal barrier protection against high glucose damage: the role of P2X7 receptor. Biochem Pharmacol 168:249–258. https://doi.org/10.1016/j.bcp.2019.07.010
Platania CBM, Maisto R, Trotta MC, D’Amico M, Rossi S, Gesualdo C, D’Amico G, Balta C, Herman H, Hermenean A, Ferraraccio F, Panarese I, Drago F, Bucolo C (2019) Retinal and circulating miRNA expression patterns in diabetic retinopathy: an in silico and in vivo approach. Br J Pharmacol 176:2179–2194. https://doi.org/10.1111/bph.14665
Boyd JM, Subramanian T, Schaeper U, La Regina M, Bayley S, Chinnadurai G (1993) A region in the C-terminus of adenovirus 2/5 E1a protein is required for association with a cellular phosphoprotein and important for the negative modulation of T24-ras mediated transformation, tumorigenesis and metastasis. EMBO J 12:469–478
Zhang C, Li S, Qiao B, Yang K, Liu R, Ma B, Liu Y, Zhang Z, Xu Y (2015) CtBP2 overexpression is associated with tumorigenesis and poor clinical outcome of prostate cancer. Arch Med Sci 11:1318–1323. https://doi.org/10.5114/aoms.2015.56359
Zhang J, Zhu J, Yang L, Guan C, Ni R, Wang Y, Ji L, Tian Y (2015) Interaction with CCNH/CDK7 facilitates CtBP2 promoting esophageal squamous cell carcinoma (ESCC) metastasis via upregulating epithelial-mesenchymal transition (EMT) progression. Tumour Biol 36:6701–6714. https://doi.org/10.1007/s13277-015-3354-x
Wang W, Zhang G, Gu H, Liu Y, Lao J, Li K, Guan H (2015) Role of CtBP2 in the apoptosis of retinal ganglion cells. Cell Mol Neurobiol 35:633–640. https://doi.org/10.1007/s10571-015-0158-x
Chinnadurai G (2003) CtBP family proteins: more than transcriptional corepressors. BioEssays 25:9–12. https://doi.org/10.1002/bies.10212
Liang H, Fekete DM, Andrisani OM (2011) CtBP2 downregulation during neural crest specification induces expression of Mitf and REST, resulting in melanocyte differentiation and sympathoadrenal lineage suppression. Mol Cell Biol 31:955–970. https://doi.org/10.1128/MCB.01062-10
Salta E, Lau P, Sala Frigerio C, Coolen M, Bally-Cuif L, De Strooper B (2014) A self-organizing miR-132/Ctbp2 circuit regulates bimodal notch signals and glial progenitor fate choice during spinal cord maturation. Dev Cell 30:423–436. https://doi.org/10.1016/j.devcel.2014.07.006
Zhang G, Kang L, Chen J, Xue Y, Yang M, Qin B, Yang L, Zhang J, Lu H, Guan H (2016) CtBP2 regulates TGFbeta2-induced epithelial-mesenchymal transition through notch signaling pathway in lens epithelial cells. Curr Eye Res 41:1057–1063. https://doi.org/10.3109/02713683.2015.1092554
Szabadfi K, Reglodi D, Szabo A, Szalontai B, Valasek A, Setalo G Jr, Kiss P, Tamas A, Wilhelm M, Gabriel R (2016) Pituitary adenylate cyclase activating polypeptide, a potential therapeutic agent for diabetic retinopathy in rats: focus on the vertical information processing pathway. Neurotox Res 29:432–446. https://doi.org/10.1007/s12640-015-9593-1
Di LJ, Byun JS, Wong MM, Wakano C, Taylor T, Bilke S, Baek S, Hunter K, Yang H, Lee M, Zvosec C, Khramtsova G, Cheng F, Perou CM, Miller CR, Raab R, Olopade OI, Gardner K (2013) Genome-wide profiles of CtBP link metabolism with genome stability and epithelial reprogramming in breast cancer. Nat Commun 4:1449. https://doi.org/10.1038/ncomms2438
Hildebrand JD, Soriano P (2002) Overlapping and unique roles for C-terminal binding protein 1 (CtBP1) and CtBP2 during mouse development. Mol Cell Biol 22:5296–5307. https://doi.org/10.1128/mcb.22.15.5296-5307.2002
Xing Q, Zhang G, Kang L, Wu J, Chen H, Liu G, Zhu R, Guan H, Lu P (2017) The suppression of kallistatin on high-glucose-induced proliferation of retinal endothelial cells in diabetic retinopathy. Ophthalmic Res 57:141–149. https://doi.org/10.1159/000447776
Chen Z, Liu G, Xiao Y, Lu P (2014) Adrenomedullin22-52 suppresses high-glucose-induced migration, proliferation, and tube formation of human retinal endothelial cells. Mol Vis 20:259–269
Kowluru RA, Kanwar M (2009) Oxidative stress and the development of diabetic retinopathy: contributory role of matrix metalloproteinase-2. Free Radic Biol Med 46:1677–1685. https://doi.org/10.1016/j.freeradbiomed.2009.03.024
Yuan L, Hu J, Luo Y, Liu Q, Li T, Parish CR, Freeman C, Zhu X, Ma W, Hu X, Yu H, Tang S (2012) Upregulation of heparanase in high-glucose-treated endothelial cells promotes endothelial cell migration and proliferation and correlates with Akt and extracellular-signal-regulated kinase phosphorylation. Mol Vis 18:1684–1695
Premanand C, Rema M, Sameer MZ, Sujatha M, Balasubramanyam M (2006) Effect of curcumin on proliferation of human retinal endothelial cells under in vitro conditions. Invest Ophthalmol Vis Sci 47:2179–2184. https://doi.org/10.1167/iovs.05-0580
Fan K, Li S, Liu G, Yuan H, Ma L, Lu P (2017) Tanshinone IIA inhibits high glucoseinduced proliferation, migration and vascularization of human retinal endothelial cells. Mol Med Rep 16:9023–9028. https://doi.org/10.3892/mmr.2017.7743
Chen YW, Paliwal S, Draheim K, Grossman SR, Lewis BC (2008) p19Arf inhibits the invasion of hepatocellular carcinoma cells by binding to C-terminal binding protein. Cancer Res 68:476–482. https://doi.org/10.1158/0008-5472.CAN-07-1960
Wang L, Zhou H, Wang Y, Cui G, Di LJ (2015) CtBP maintains cancer cell growth and metabolic homeostasis via regulating SIRT4. Cell Death Dis 6:e1620. https://doi.org/10.1038/cddis.2014.587
Takayama K, Suzuki T, Fujimura T, Urano T, Takahashi S, Homma Y, Inoue S (2014) CtBP2 modulates the androgen receptor to promote prostate cancer progression. Cancer Res 74:6542–6553. https://doi.org/10.1158/0008-5472.CAN-14-1030
Sumner ET, Chawla AT, Cororaton AD, Koblinski JE, Kovi RC, Love IM, Szomju BB, Korwar S, Ellis KC, Grossman SR (2017) Transforming activity and therapeutic targeting of C-terminal-binding protein 2 in Apc-mutated neoplasia. Oncogene 36:4810–4816. https://doi.org/10.1038/onc.2017.106
Jin L, Zhang Y, Liang W, Lu X, Piri N, Wang W, Kaplan HJ, Dean DC, Zhang L, Liu Y (2020) Zeb1 promotes corneal neovascularization by regulation of vascular endothelial cell proliferation. Commun Biol 3:349. https://doi.org/10.1038/s42003-020-1069-z
Tsai CH, Chiang YC, Chen HT, Huang PH, Hsu HC, Tang CH (2013) High glucose induces vascular endothelial growth factor production in human synovial fibroblasts through reactive oxygen species generation. Biochim Biophys Acta 1830:2649–2658. https://doi.org/10.1016/j.bbagen.2012.12.017
Graupera M, Guillermet-Guibert J, Foukas LC, Phng LK, Cain RJ, Salpekar A, Pearce W, Meek S, Millan J, Cutillas PR, Smith AJ, Ridley AJ, Ruhrberg C, Gerhardt H, Vanhaesebroeck B (2008) Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature 453:662–666. https://doi.org/10.1038/nature06892
Karar J, Maity A (2011) PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci 4:51. https://doi.org/10.3389/fnmol.2011.00051
Paliwal S, Kovi RC, Nath B, Chen YW, Lewis BC, Grossman SR (2007) The alternative reading frame tumor suppressor antagonizes hypoxia-induced cancer cell migration via interaction with the COOH-terminal binding protein corepressor. Cancer Res 67:9322–9329. https://doi.org/10.1158/0008-5472.CAN-07-1743
Wang DP, Gu LL, Xue Q, Chen H, Mao GX (2018) CtBP2 promotes proliferation and reduces drug sensitivity in non-small cell lung cancer via the Wnt/beta-catenin pathway. Neoplasma 65:888–897. https://doi.org/10.4149/neo_2018_171220N828
Easwaran V, Lee SH, Inge L, Guo L, Goldbeck C, Garrett E, Wiesmann M, Garcia PD, Fuller JH, Chan V, Randazzo F, Gundel R, Warren RS, Escobedo J, Aukerman SL, Taylor RN, Fantl WJ (2003) beta-Catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Res 63:3145–3153
Mack JJ, Iruela-Arispe ML (2018) NOTCH regulation of the endothelial cell phenotype. Curr Opin Hematol 25:212–218. https://doi.org/10.1097/MOH.0000000000000425
Liu L, Tong Q, Liu S, Cui J, Zhang Q, Sun W, Yang S (2016) ZEB1 Upregulates VEGF expression and stimulates angiogenesis in breast Cancer. PLoS ONE 11:e0148774. https://doi.org/10.1371/journal.pone.0148774
Postigo AA, Dean DC (1999) ZEB represses transcription through interaction with the corepressor CtBP. Proc Natl Acad Sci U S A 96:6683–6688. https://doi.org/10.1073/pnas.96.12.6683
Nishijima K, Ng YS, Zhong L, Bradley J, Schubert W, Jo N, Akita J, Samuelsson SJ, Robinson GS, Adamis AP, Shima DT (2007) Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol 171:53–67. https://doi.org/10.2353/ajpath.2007.061237
Rangasamy S, McGuire PG, Das A (2012) Diabetic retinopathy and inflammation: novel therapeutic targets. Middle East Afr J Ophthalmol 19:52–59. https://doi.org/10.4103/0974-9233.92116
Kong Y, Naggert JK, Nishina PM (2018) The impact of adherens and tight junctions on physiological function and pathological changes in the retina. Adv Exp Med Biol 1074:545–551. https://doi.org/10.1007/978-3-319-75402-4_66
Mugisho OO, Green CR, Zhang J, Acosta ML, Rupenthal ID (2019) Connexin43 hemichannels: a potential drug target for the treatment of diabetic retinopathy. Drug Discov Today 24:1627–1636. https://doi.org/10.1016/j.drudis.2019.01.011
Thio SS, Bonventre JV, Hsu SI (2004) The CtBP2 co-repressor is regulated by NADH-dependent dimerization and possesses a novel N-terminal repression domain. Nucleic Acids Res 32:1836–1847. https://doi.org/10.1093/nar/gkh344
Minard ME, Kim LS, Price JE, Gallick GE (2004) The role of the guanine nucleotide exchange factor Tiam1 in cellular migration, invasion, adhesion and tumor progression. Breast Cancer Res Treat 84:21–32. https://doi.org/10.1023/B:BREA.0000018421.31632.e6
Li B, Song Y, Liu TJ, Cui YB, Jiang Y, Xie ZS, Xie SL (2013) miRNA-22 suppresses colon cancer cell migration and invasion by inhibiting the expression of T-cell lymphoma invasion and metastasis 1 and matrix metalloproteinases 2 and 9. Oncol Rep 29:1932–1938. https://doi.org/10.3892/or.2013.2300
Paliwal S, Ho N, Parker D, Grossman SR (2012) CtBP2 promotes human cancer cell migration by transcriptional activation of tiam1. Genes Cancer 3:481–490. https://doi.org/10.1177/1947601912463695
Acknowledgements
The abstract of this manuscript has been presented in Abstracts from “The 24th Congress of Chinese Ophthalmological Society Suzhou, China September 4-8, 2019”.
Funding
This study was funded by the National Natural Science Foundation of China (81500706), the Postdoctoral Science Foundation of China (2020M671562), the Postdoctoral Science Foundation of Jiangsu Province (2020Z318), the Science and Technology Project of Nantong Municipality (YYZ17010 and HS2020005).
Author information
Authors and Affiliations
Contributions
XC designed this research. HL, FZ and XC performed experiments and drafted this manuscript. PZ and XH collected tissues samples and analyzed clinical data. WX assisted with part of cell and animal experiments. GZ edited the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, H., Zhou, F., Cao, X. et al. C-terminal binding protein 2 promotes high-glucose-triggered cell proliferation, angiogenesis and cellular adhesion of human retinal endothelial cell line. Int Ophthalmol 42, 2975–2985 (2022). https://doi.org/10.1007/s10792-022-02283-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-022-02283-9