Abstract
Background
Inflammation is a key biological reaction that comprises a complex network of signals that both initiate and stop the inflammation process.
Purpose
This study targets to evaluate the anti-inflammatory potential of the leaves of the Plectranthus rugosus (P. rugosus) plant involving both in vitro and in vivo measures. The current available drugs exhibit serious side effects. Traditional medicines impart an essential role in drug development. P. rugosus is a plant used in traditional medicine of Tropical Africa, China, and Australia to treat various diseases.
Methods
Lipopolysaccharide (LPS), an endotoxin, kindles macrophages to discharge huge quantities of pro-inflammatory cytokines like TNF-α and IL-6. So, clampdown of macrophage stimulation may have a beneficial potential to treat various inflammatory disorders. The leaves of the P. rugosus are used for swelling purpose by local population; however, its use as an anti-inflammatory agent and associated disorders has no scientific evidence.
Results
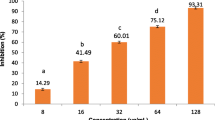
The extracts of the plant Plectranthus rugosus ethanolic extract (PREE), Plectranthus rugosus ethyl acetate extract (PREAF), and the compound isolated (oleanolic acid) suppress the pro-inflammatory cytokines (IL-6 and TNF-α) and nitric oxide (NO), confirming its importance in traditional medicine.
Conclusion
The pro-inflammatory cytokines are inhibited by P. rugosus extracts, as well as an isolated compound oleanolic acid without compromising cell viability.











Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article.
References
Adnan M, Begum S, Khan AL, Tareen AM, Lee I-J (2012a) Medicinal plants and their uses in selected temperate zones of Pakistani Hindukush-Himalaya. J Med Plants Res 6:4113–4127
Adnan M, Begum S, Latif A, Tareen AM, Lee L (2012b) Medicinal plants and their uses in selected temperate zones of Pakistani Hindukush-Himalaya. J Med Plants Res 6:4113–4127
Affendi Raja Ali R, John EL (2011) How to manage the risk of colorectal cancer in ulcerative colitis. Curr Drug Targets 12:1424–1432
Ahmad M, Sultana S, Fazl-I-Hadi S, Ben Hadda T, Rashid S, Zafar M, Khan MA, Khan MPZ, Yaseen G (2014) An ethnobotanical study of medicinal plants in high mountainous region of Chail valley (District Swat-Pakistan). J Ethnobiol Ethnomed 10:36
Akhtar N, Rashid A, Murad W, Bergmeier E (2013) Diversity and use of ethno-medicinal plants in the region of Swat, North Pakistan. J Ethnobiol Ethnomed 9:25
Ammon H (2010) Modulation of the immune system by Boswellia serrata extracts and boswellic acids. Phytomedicine 17:862–867
Ananthi S, Raghavendran HRB, Sunil AG, Gayathri V, Ramakrishnan G, Vasanthi HR (2010) In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide from Turbinaria ornata (Marine Brown Alga). Food Chem Toxicol 48:187–192
Barnes PJ (2006) How corticosteroids control inflammation: quintiles prize lecture 2005. Br J Pharmacol 148:245–254
Barton GM (2008) A calculated response: control of inflammation by the innate immune system. J Clin Invest 118:413–420
Braca A, de Tommasi N, di Bari L, Pizza C, Politi M, Morelli I (2001) Antioxidant principles from bauhinia t arapotensis. J Nat Prod 64:892–895
Charles JF, Humphrey MB, Zhao X, Quarles E, Nakamura MC, Aderem A, Seaman WE, Smith KD (2008) The innate immune response to Salmonella enterica serovar Typhimurium by macrophages is dependent on TREM2-DAP12. Infect Immun 76:2439–2447
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L (2018) Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9:7204
Cotran R (1999) Cellular pathology I: cell injury and cell death. In: Robbins pathologic basis of disease, pp 23–25
Duncan FJ, Wulff BC, Tober KL, Ferketich AK, Martin J, Thomas-Ahner JM, Allen SD, Kusewitt DF, Oberyszyn TM, Vanbuskirk AM (2007) Clinically relevant immunosuppressants influence UVB-induced tumor size through effects on inflammation and angiogenesis. Am J Transplant 7:2693–2703
Dunn DL, Barke RA, Ewald DC, Simmons RL (1987) Macrophages and translymphatic absorption represent the first line of host defense of the peritoneal cavity. Arch Surg 122:105–110
Ernst E (2000) Prevalence of use of complementary/alternative medicine: a systematic review. Bullet World Health Organ 78:258–266
Folmer F, Jaspars M, Dicato M, Diederich M (2008) Marine natural products as targeted modulators of the transcription factor NF-κB. Biochem Pharmacol 75:603–617
Freire MO, van Dyke TE (2013) Natural resolution of inflammation. Periodontol 2000(63):149–164
Fujiwara N, Kobayashi K (2005) Macrophages in inflammation. Curr Drug Targets-Inflam Allergy 4:281–286
Gautam R, Jachak SM (2009) Recent developments in anti-inflammatory natural products. Med Res Rev 29:767–820
Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K (2010) Development of monocytes, macrophages, and dendritic cells. Science 327:656–661
Grabley S, Sattler I (2003) Natural products for lead identification: nature is a valuable resource for providing tools. In: Hillisch A, Hilgenfeld R (eds) Modern methods of drug discovery. Springer, Basel
Ho KY, Gwee KA, Cheng YK, Yoon KH, Hee HT, Omar AR (2018) Nonsteroidal anti-inflammatory drugs in chronic pain: implications of new data for clinical practice. J Pain Res. https://doi.org/10.2147/JPR.S168188
Hortelano S (2009) Molecular basis of the anti-inflammatory effects of terpenoids. Inflamm Allergy-Drug Targets 8:28–39
Hosseinzadeh H, Younesi HM (2002) Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol 2:7
Hosseinzadeh H, Ramezani M, Salmani G-A (2000) Antinociceptive, anti-inflammatory and acute toxicity effects of Zataria multiflora Boiss extracts in mice and rats. J Ethnopharmacol 73:379–385
Hou J, Karin M, Sun BJNRCO (2021) Targeting cancer-promoting inflammation—have anti-inflammatory therapies come of age? Nat Rev Clin Oncol 18:261–279
Irshad M, Aziz S, Habib-Ur-Rehman Hussain H (2012) GC-MS analysis and antifungal activity of essential oils of angelica glauca, plectranthus rugosus, and Valeriana wallichii. J Essent Oil Bear Plants 15:15–21
Joo T, Sowndhararajan K, Hong S, Lee J, Park S-Y, Kim S, Jhoo J-W (2014) Inhibition of nitric oxide production in LPS-stimulated RAW 264.7 cells by stem bark of Ulmus pumila L. Saudi J Biol Sci 21:427–435
Kala CP, Farooquee NA, Dhar U (2004) Prioritization of medicinal plants on the basis of available knowledge, existing practices and use value status in Uttaranchal, India. Biodivers Conserv 13:453–469
Karin M, Greten FR (2005) NF-κB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 5:749–759
Khan SW, Khatoon S (2007) Ethnobotanical studies on useful trees and shrubs of Haramosh and Bugrote valleys in Gilgit northern areas of Pakistan. Pak J Bot 39:699–710
Lawrence T, Fong C (2010) The resolution of inflammation: anti-inflammatory roles for NF-κB. Int J Biochem Cell Biol 42:519–523
Lee KH (2010) Discovery and development of natural product-derived chemotherapeutic agents based on a medicinal chemistry approach. J Natl Prod 73:500–516
Li G, Lou HX (2018) Strategies to diversify natural products for drug discovery. Med Res Rev 38:1255–1294
Libby P (2002) Ridker PM, and Maseri A. Inflam Atheroscler Circ 105:1135–1143
Lin W-C, Lin J-Y (2010) Five bitter compounds display different anti-inflammatory effects through modulating cytokine secretion using mouse primary splenocytes in vitro. J Agric Food Chem 59:184–192
Lukhoba CW, Simmonds MS, Paton AJ (2006) Plectranthus: a review of ethnobotanical uses. J Ethnopharmacol 103:1–24
Mahesh G, Anil Kumar K, Reddanna P (2021) Overview on the discovery and development of anti-inflammatory drugs: should the focus be on synthesis or degradation of PGE2? J Inflam Res. https://doi.org/10.2147/JIR.S278514
Mir RH, Masoodi MHJCBC (2020) Anti-inflammatory plant polyphenolics and cellular action mechanisms. Curr Bioactive Compounds 16:809–817
Mir RH, Shah AJ, Mohi-Ud-din R, Pottoo FH, Dar M, Jachak SM, Masoodi MH (2021) Natural anti-inflammatory compounds as drug candidates in Alzheimer’s disease. Curr Med Chem 28:4799–4825
Mir RH, Banday N, Sabreen S, Shah AJ, Jan R, Wani TU, Farooq S, Bhat Z (2022) Resveratrol: a potential drug candidate with multispectrum therapeutic application. Stud Natl Prod Chem 73:99–137
Moore RA, Derry S, Phillips CJ, Mcquay H (2006) Nonsteroidal anti-inflammatory drugs (NSAIDs), cyxlooxygenase-2 selective inhibitors (coxibs) and gastrointestinal harm: review of clinical trials and clinical practice. BMC Musculoskelet Disord 7:1–13
Nance CL (2015) Clinical efficacy trials with natural products and herbal medicines. In: Ramzan I (ed) Phytotherapies: efficacy, safety and regulation. Wiley online library, Hoboken
Nathan C, Ding AJC (2010) Nonresolving inflammation. Cell 140:871–882
Newman DJ, Cragg GM (2007) Natural products as sources of new drugs over the last 25 years. J Nat Prod 70:461–477
Patwardhan B (2005) Ethnopharmacology and drug discovery. J Ethnopharmacol 100:50–52
Patwardhan B, Gautam M (2005) Botanical immunodrugs: scope and opportunities. Drug Discov Today 10:495–502
Perrone S, Lotti F, Geronzi U, Guidoni E, Longini M, Buonocore G (2016) Oxidative stress in cancer-prone genetic diseases in pediatric age: the role of mitochondrial dysfunction. Oxid Med Cell Long. https://doi.org/10.1155/2016/4782426
Rainsford K (1999) Profile and mechanisms of gastrointestinal and other side effects of nonsteroidal anti-inflammatory drugs (NSAIDs). Am J Med 107:27–35
Rao P, Knaus EE (2008) Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J Pharm Pharm Sci 11:81–110s
Razdan T, Kachroo V, Harkar S, Koul G, Dhart KJP (1982) Plectranthoic acid, acetylplectranthoic acid and plectranthadiol, three triterpenoids from Plectranthus rugosus. Phytochemistry 21:409–412
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med 26:1231–1237
Sabeen M, Ahmad SS (2009) Exploring the folk medicinal flora of Abbotabad city, Pakistan. Ethnobotanical Leaflets 2009:1
Scharl M, Rogler G (2012) Inflammatory bowel disease: dysfunction of autophagy? Dig Dis 30:12–19
Schett G (2006) Rheumatoid arthritis: inflammation and bone loss. Wien Med Wochenschr 156:34–41
Shah AJ, Mir RH, Pottoo FH, Masoodi MH, Bhat ZA (2021) Depression: an insight into heterocyclic and cyclic hydrocarbon compounds inspired from natural sources. Curr Neuropharmacol. https://doi.org/10.2174/1570159X19666210426115234
Shaikh RU, Pund MM, Gacche RN, Mediciney C (2016) Evaluation of anti-inflammatory activity of selected medicinal plants used in Indian traditional medication system in vitro as well as in vivo. J Tradit Complement Med 6:355–361
Shuaib M, Khan I, Sharifullah RK, Hashmatullah SM, Naz R (2014) Ethnobotanical studies of spring flora of Dir Lower, Khyber Pakhtunkhwa, Pakistan. Pak J Weed Sci Res 20:37–49
Shuaib M, Khan I, Sharifullah KM (2015) Study of medicinal plants of lower dir, Timergara, Tehsil Balambat, Khyber Paktunkhaw-Pakistan. Am Eurasian J Agric Environ Sci 15:2088–2094
Sibilia J (2003) Corticosteroids and inflammation. Rev Prat 53:495–501
Singh P, Kumar R, Prakash O, Pant AK, Kumar M, Isidorov VA, Szczepaniak LJ. 2019. Chemical composition, anti-inflammatory, analgesic, antipyretic, myorelaxant, antibacterial and antifungal activity of rabdosia rugosus wall.(Syn. Plectranthus rugosus Wall.). 5, 8–15.
Stark K, Massberg S (2021) Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol 18:666–682
Tiwari A, Padalia R, Mathela CJ (2008) Sesquiterpene rich essential oil from plectranthus rugosus wall. J Essent Oil BearPlants 11:58–61
Tuchscherer M, Otten W, Kanitz E, Gräbner M, Tuchscherer A, Bellmann O, Rehfeldt C, Metges CC (2012) Effects of inadequate maternal dietary protein: carbohydrate ratios during pregnancy on offspring immunity in pigs. BMC Vet Res 8:232
Wahyuni IS, Sufiawati I, Nittayananta W, Levita J (2022) Anti-inflammatory activity and wound healing effect of Kaempferia galanga L. Rhizome on the chemical-induced oral mucosal ulcer in wistar rats. J Inflam Res. https://doi.org/10.2147/JIR.S359042
Weyerstahl P, Kaul V, Meier N, Weirauch M, Marschall H (1983a) Volatile constituents of Plectranthus rugosus leaf oil. Planta Med 48:99–102
Weyerstahl P, Kaul V, Meier N, Weirauch M, Marschall HJPM (1983b) Volatile constituents of Plectranthus rugosus leaf oil. J Essential Oil Bear Plants 48:99–102
Whelton A (2000) Renal and related cardiovascular effects of conventional and COX-2-specific NSAIDs and non-NSAID analgesics. Am J Therap 7:63–74
Xu X, Yin P, Wan C, Chong X, Liu M, Cheng P, Chen J, Liu F, Xu J (2014) Punicalagin inhibits inflammation in LPS-induced RAW264. 7 macrophages via the suppression of TLR4-mediated MAPKs and NF-κB activation. Inflammation 37:956–965
Acknowledgements
Lamya Ahmed Al-Keridis extends her appreciation to Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2024R82), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Funding
This work is supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2024R82), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
RHM and MHM contributed to study concept and design, collected/analyzed data, performed project administration, and drafted the original manuscript. RM-ud-d, LAAl-K, BA, NA, MP, and MA contributed to methodology, data curation, investigation, visualization, analysis, review, and editing; RHM, MA, MHM, and RM-ud-d contributed to critical revision of the manuscript, methodology, validation, formal analysis, and study supervision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
The protocol for the experiment was approved by the Institutional Animal ethics committee (Registration No. 801/GO/Re/2003/CPCSEA), University of Kashmir, India.
Informed consent statement
Not applicable.
Conflicts of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mir, R.H., Mohi-ud-din, R., Al-Keridis, L.A. et al. Phytochemical profiling, antioxidant, cytotoxic, and anti-inflammatory activities of Plectranthus rugosus extract and fractions: in vitro, in vivo, and in silico approaches. Inflammopharmacol 32, 1593–1606 (2024). https://doi.org/10.1007/s10787-023-01419-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-023-01419-2