Abstract
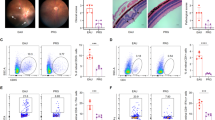
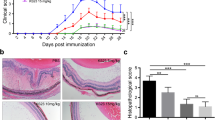
4-octyl itaconate (4-OI) is an anti-inflammatory metabolite that activates the nuclear-factor-E2-related factor 2 (NRF2) signaling. In the current work, we investigated whether 4-OI could affect the production of proinflammatory cytokines in Behcet’s uveitis (BU) and experimental autoimmune uveitis (EAU). Peripheral blood mononuclear cells (PBMCs) of active BU patients and healthy individuals with in vitro 4-OI treatment were performed to assess the influence of 4-OI on the proinflammatory cytokine production. EAU was induced and used for investigating the influence of 4-OI on the proinflammatory cytokine production in vivo. The flow cytometry, qPCR, and ELISA were performed to detect proinflammatory cytokine expression. NRF2 signaling activation was evaluated by qPCR and western blotting (WB). Splenic lymphocyte transcriptome was performed by RNA sequencing. The NRF2 expression by BU patients-derived PBMCs was lower than that by healthy individuals. After treatment with 4-OI, the proportion of Th17 cells, along with the expression of proinflammatory cytokines (IL-17, TNF-α, MCP-1, and IL-6) by PBMCs, were downregulated, and anti-inflammatory cytokine (IL-10) expression was upregulated, although IFN-γ expression was unaffected. The EAU severity was ameliorated by 4-OI in association with a lower splenic Th1/Th17 cell proportion and increased nuclear NRF2 expression. Additionally, 4-OI downregulated a set of 248 genes, which were enriched in pathways of positive regulation of immune responses. The present study shows an inhibitory effect of 4-OI on the proinflammatory cytokine production in active BU patients and EAU mice, possibly mediated through activating NRF2 signaling. These findings suggest that 4-OI could act as a potential therapeutic drug for the treatment and prevention of BU in the future study.






Similar content being viewed by others
Availability of Data and Material
The data sets used and/or analyzed in this study are available from the corresponding authors upon reasonable request. The dataset of RNA-seq is available in https://www.jianguoyun.com/, and the shared data link is https://www.jianguoyun.com/p/DRZEH24Qn5H8CxjW55sFIAA.
References
Mendes, D., et al. 2009. Behcet’s disease–a contemporary review. Journal of Autoimmunity 32 (3–4): 178–188.
Kitaichi, N., et al. 2009. Low prevalence of juvenile-onset Behcet’s disease with uveitis in East/South Asian people. British Journal of Ophthalmology 93 (11): 1428–1430.
Lu, A., et al. 2023. Global research status regarding uveitis in the last decade. Ocular Immunology and Inflammation 1–10.
Ben Ahmed, M., et al. 2004. Involvement of chemokines and Th1 cytokines in the pathogenesis of mucocutaneous lesions of Behcet’s disease. Arthritis and Rheumatism 50 (7): 2291–2295.
Kim, J., et al. 2010. Imbalance of Th17 to Th1 cells in Behcet’s disease. Clinical and Experimental Rheumatology 28 (4 Suppl 60): S16–S19.
Zhong, Z., et al. 2021. Activation of the interleukin-23/interleukin-17 signalling pathway in autoinflammatory and autoimmune uveitis. Progress in Retinal and Eye Research 80: 100866.
Tong, B., et al. 2019. Immunopathogenesis of Behcet’s disease. Frontiers of Immunology 10: 665.
Chen, J., et al. 2022. 7-deacetyl-gedunin suppresses proliferation of human rheumatoid arthritis synovial fibroblast through activation of Nrf2/ARE signaling. International Immunopharmacology 107: 108557.
Puppala, E.R., et al. 2022. Perillyl alcohol attenuates rheumatoid arthritis via regulating TLR4/NF-kappaB and Keap1/Nrf2 signaling pathways: A comprehensive study on in-vitro and in-vivo experimental models. Phytomedicine 97: 153926.
Su, X., et al. 2021. Artesunate attenuates bone erosion in rheumatoid arthritis by suppressing reactive oxygen species via activating p62/Nrf2 signaling. Biomedicine & Pharmacotherapy 137: 111382.
Chadha, S., et al. 2020. Role of Nrf2 in rheumatoid arthritis. Current Research in Translational Medicine 68 (4): 171–181.
Barati, M.T., and D.J. Caster. 2022. The potential of Nrf2 activation as a therapeutic target in systemic lupus erythematosus. Metabolites 12 (2).
Han, S., et al. 2020. NF-E2-related factor 2 regulates interferon receptor expression and alters macrophage polarization in lupus. Arthritis & Rhematology 72 (10): 1707–1720.
Gautam, P., et al. 2022. Proportion of B cell subsets and Nrf2 mediated redox regulation in systemic lupus erythematosus patients. Immunobiology 227 (2): 152180.
Johnson, D.A., et al. 2010. The absence of the pro-antioxidant transcription factor Nrf2 exacerbates experimental autoimmune encephalomyelitis. Toxicological Sciences 114 (2): 237–246.
Strelko, C.L., et al. 2011. Itaconic acid is a mammalian metabolite induced during macrophage activation. Journal of the American Chemical Society 133 (41): 16386–16389.
Mills, E.L., et al. 2018. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 556 (7699): 113–117.
Runtsch, M.C., et al. 2022. Itaconate and itaconate derivatives target JAK1 to suppress alternative activation of macrophages. Cell Metabolism 34 (3): 487–501 e8.
Hooftman, A., et al. 2020. The immunomodulatory metabolite itaconate modifies NLRP3 and inhibits inflammasome activation. Cell Metabolism 32 (3): 468–478 e7.
Jaiswal, A.K., et al. 2022. Irg1/itaconate metabolic pathway is a crucial determinant of dendritic cells immune-priming function and contributes to resolute allergen-induced airway inflammation. Mucosal Immunology 15 (2): 301–313.
The International Criteria for Behcet's Disease (ICBD). 2014. a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. Journal of the European Academy of Dermatology and Venereology 28 (3): 338–47.
Hu, B., et al. 2020. Nuclear factor E2 related factor (NRF2) inhibits mast cell- mediated allergic inflammation via SIRT4-mediated mitochondrial metabolism. Annals of Palliative Medicine 9 (6): 3839–3847.
Mattapallil, M.J., et al. 2015. Characterization of a new epitope of IRBP that induces moderate to severe uveoretinitis in mice with H-2b haplotype. Investigative Ophthalmology & Visual Science 56 (9): 5439–5449.
Song, H., et al. 2020. Itaconate prevents abdominal aortic aneurysm formation through inhibiting inflammation via activation of Nrf2. eBioMedicine 57: 102832.
Cortes, L.M., et al. 2008. Repertoire analysis and new pathogenic epitopes of IRBP in C57BL/6 (H-2b) and B10.RIII (H-2r) mice. Investigative Ophthalmology and Visual Science 49 (5): 1946–1956.
Yi, S., et al. 2018. Disabled-2 (DAB2) Overexpression inhibits monocyte-derived dendritic cells’ function in Vogt-Koyanagi-Harada disease. Investigative Ophthalmology & Visual Science 59 (11): 4662–4669.
Ross, D., and D. Siegel. 2021. The diverse functionality of NQO1 and its roles in redox control. Redox Biology 41: 101950.
Yu, M., et al. 2013. Nrf2/ARE is the potential pathway to protect Sprague-Dawley rats against oxidative stress induced by quinocetone. Regulatory Toxicology and Pharmacology 66 (3): 279–285.
Gorrini, C., I.S. Harris, and T.W. Mak. 2013. Modulation of oxidative stress as an anticancer strategy. Nature Reviews. Drug Discovery 12 (12): 931–947.
Gautam, P., et al. 2020. Altered redox regulation by Nrf2-Keap1 system in dendritic cells of systemic lupus erythematosus patients. Lupus 29 (12): 1544–1555.
Bozkurt, M., et al. 2014. Serum prolidase enzyme activity and oxidative status in patients with Behcet’s disease. Redox Report 19 (2): 59–64.
Leccese, P., and E. Alpsoy. 2019. Behcet’s disease: An overview of etiopathogenesis. Frontiers in Immunology 10: 1067.
Yu, C., et al. 2021. Astragaloside IV-induced Nrf2 nuclear translocation ameliorates lead-related cognitive impairments in mice. Biochimica et Biophysica Acta, Molecular Cell Research 1868 (1): 118853.
Tang, C., et al. 2018. 4-octyl itaconate activates Nrf2 signaling to inhibit pro-inflammatory cytokine production in peripheral blood mononuclear cells of systemic lupus erythematosus patients. Cellular Physiology and Biochemistry 51 (2): 979–990.
Wang, L., and C. He. 2022. Nrf2-mediated anti-inflammatory polarization of macrophages as therapeutic targets for osteoarthritis. Frontiers in Immunology 13: 967193.
Piantadosi, C.A., and H.B. Suliman. 2012. Transcriptional control of mitochondrial biogenesis and its interface with inflammatory processes. Biochimica et Biophysica Acta 1820 (4): 532–541.
Sena, L.A., et al. 2013. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38 (2): 225–236.
Ohl, K., and K. Tenbrock. 2021. Oxidative stress in SLE T cells, is NRF2 really the target to treat? Frontiers in Immunology 12: 633845.
Caspi, R.R. 2010. A look at autoimmunity and inflammation in the eye. The Journal of Clinical Investigation 120 (9): 3073–3083.
Kuo, P.C., et al. 2020. Dimethyl itaconate, an itaconate derivative, exhibits immunomodulatory effects on neuroinflammation in experimental autoimmune encephalomyelitis. Journal of Neuroinflammation 17 (1): 138.
Ko, M.K., et al. 2020. CD73(+) dendritic cells in cascading Th17 responses of experimental autoimmune uveitis-induced mice. Frontiers in Immunology 11: 601272.
Liu, X., et al. 2013. Higher expression of Toll-like receptors 2, 3, 4, and 8 in ocular Behcet’s disease. Investigative Ophthalmology & Visual Science 54 (9): 6012–6017.
Ture-Ozdemir, F., et al. 2013. Pro-inflammatory cytokine and caspase-1 responses to pattern recognition receptor activation of neutrophils and dendritic cells in Behcet’s disease. Rheumatology (Oxford) 52 (5): 800–805.
Xu, M., et al. 2020. Dimethyl itaconate protects against lipopolysaccharide-induced endometritis by inhibition of TLR4/NF-kappaB and activation of Nrf2/HO-1 signaling pathway in mice. Iranian Journal of Basic Medical Sciences 23 (9): 1239–1244.
Li, L., et al. 2016. Genetic Variations of NLR family genes in Behcet’s disease. Science and Reports 6: 20098.
Singh, S., et al. 2021. Integrative metabolomics and transcriptomics identifies itaconate as an adjunct therapy to treat ocular bacterial infection. Cell Rep Med 2 (5): 100277.
Marks, K.E., et al. 2021. Toll-like receptor 2 induces pathogenicity in Th17 cells and reveals a role for IPCEF in regulating Th17 cell migration. Cell Reports 35 (13): 109303.
Hatscher, L., et al. 2021. Select hyperactivating NLRP3 ligands enhance the TH1- and TH17-inducing potential of human type 2 conventional dendritic cells. Science Signaling 14 (680).
Acknowledgements
We appreciate the technical support provided by our laboratory’s entire clinical research personnel and students. We also appreciate the cooperation and informed consent provided by the healthy donors and patients for this study.
Funding
This study was supported by the National Natural Science Foundation Key Program (82230032), National Natural Science Foundation Key Program (81930023), Natural Science Foundation Major International (Regional) Joint Research Project (81720108009), Key Project of Chongqing Science and Technology Bureau (CSTC2021jscx-gksb-N0010), National Natural Science Foundation (82101106), Foundation of Chongqing (cstc2021jcyj-bshX0154), Chongqing Outstanding Scientists Project (2019), Chongqing Chief Medical Scientist Project (2018), Chongqing Key Laboratory of Ophthalmology (CSTC, 2008CA5003), and Chongqing Science & Technology Platform and Base Construction Program (cstc2014pt-sy10002).
Author information
Authors and Affiliations
Contributions
QW and PY: conceived and directed the study. QW, XY, ST, HL, QJ, and SP: analyzed the data. PY: made the clinical diagnoses. QC: collected the samples. QW, XY, ST, and HL: performed the in vitro experiment and animal experiment. QW and XY: evaluated histology slides. QW and GS: drafted the manuscript. PY: reviewed the data interpretation and helped revise the final versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of Chongqing Medical University (2018–048). All procedures were carried out in accordance with the Declaration of Helsinki. All participants signed an informed consent form at the start of the study. The animal experiments were approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10753_2023_1950_MOESM2_ESM.tif
Supplementary file2: The effects of 4-OI on the Th1/Th17 index cytokines as well as proinflammatory and anti-inflammatory cytokines mRNA expression by retinas of EAU mice. (A) Comparison of IFN-γ and IL-17 mRNA expression between 4-OI-treated group and control group. (B) Comparison of IL-6, TNF-α, MCP-1and IL-10 mRNA expression between 4-OI-treated group and control group. β-actin was used as the internal reference. Fold change is used to show the gene expression in the 4-OI-treated group relative to that in the control group. Data were represented as the relative fold change (2−∆∆Ct). Data were analyzed by unpaired t-test (n = 7 per group). *p < 0.05; **p < 0.01. (TIF 106 KB)
10753_2023_1950_MOESM3_ESM.tif
Supplementary file3: Validation of five DEGs from the RNA-seq data. β-actin was used as the internal reference. Fold change is used to show the gene expression in the 4-OI-treated group relative to that in the control group. Data were represented as the relative fold change (2−∆∆Ct). Data were analyzed by unpaired t-test (n = 7 per group). *p < 0.05; **p < 0.01. (TIF 90 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Q., Ye, X., Tan, S. et al. 4-Octyl Itaconate Inhibits Proinflammatory Cytokine Production in Behcet’s Uveitis and Experimental Autoimmune Uveitis. Inflammation (2024). https://doi.org/10.1007/s10753-023-01950-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10753-023-01950-y