Abstract
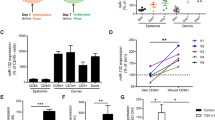
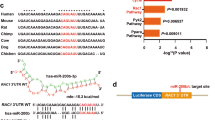
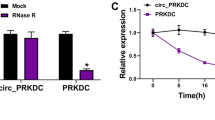
The management of skin wound healing is still a challenge. MicroRNA-21 (miR-21) has been reported to play important roles in wound repair; however, the underlying mechanism needs to be further clarified. The present study aimed to study the direct role of miR-21 in skin wound healing in miR-21 KO mice and to investigate the role of miR-21 in controlling the migration and proliferation of primary human skin cells and its underlying mechanism(s). miR-21 KO and wild-type (WT) mice were used for in vivo wound healing assays, while mouse and human primary skin cells were used for in vitro assays. miR-21 inhibitors or mimics or negative control small RNAs were transfected to either inhibit or enhance miR-21 expression in the human primary dermal fibroblasts or epidermal cells. RNA sequencing analysis was performed to identify the potential molecular pathways involved. We found that the loss of miR-21 resulted in slower wound healing in miR-21 KO mouse skin and especially delayed the healing of dermal tissue. In vitro assays demonstrated that the reduced expression of miR-21 caused by its inhibitor inhibited the migration of human primary dermal fibroblasts, which could be enhanced by increased miR-21 expression caused by miR-21 mimics. RNA-sequence analysis revealed that the inhibition of miR-21 expression downregulated the inflammatory response pathways associated with the decreased expression of inflammatory cytokines, and the addition of IL-1β into the culture medium enhanced the migration and proliferation of dermal fibroblasts in vitro. In conclusion, miR-21 in dermal fibroblasts can promote the migration and growth of epidermal and dermal cells to enhance skin wound healing through controlling the expression of inflammatory cytokines.






Similar content being viewed by others
Data Availability
Datasets are available on request.
References
Sorg, H., et al. 2018. Panta Rhei: neovascularization, angiogenesis and nutritive perfusion in wound healing. European Surgical Research 59 (3–4): 232–241.
Wojtowicz, A.M., et al. 2014. The importance of both fibroblasts and keratinocytes in a bilayered living cellular construct used in wound healing. Wound Repair and Regeneration 22 (2): 246–255.
Werner, S., T. Krieg, and H. Smola. 2007. Keratinocyte-fibroblast interactions in wound healing. The Journal of Investigative Dermatology 127 (5): 998–1008.
Li, B., and J.H. Wang. 2011. Fibroblasts and myofibroblasts in wound healing: force generation and measurement. Journal of Tissue Viability 20 (4): 108–120.
Stunova, A., and L. Vistejnova. 2018. Dermal fibroblasts-a heterogeneous population with regulatory function in wound healing. Cytokine & Growth Factor Reviews 39: 137–150.
Tracy, L.E., R.A. Minasian, and E.J. Caterson. 2016. Extracellular matrix and dermal fibroblast function in the healing wound. Advances in Wound Care 5 (3): 119–136.
Darby, I.A., et al. 2014. Fibroblasts and myofibroblasts in wound healing. Clinical, Cosmetic and Investigational Dermatology 7: 301–311.
Cooper, P.O., et al. 2021. Dermal drivers of injury-induced inflammation: contribution of adipocytes and fibroblasts. International Journal of Molecular Sciences 22 (4): 1933.
Werner, S., and R. Grose. 2003. Regulation of wound healing by growth factors and cytokines. Physiological Reviews 83 (3): 835–870.
Bainbridge, P. 2013. Wound healing and the role of fibroblasts. Journal of Wound Care 22 (8): 407–408, 410–412.
Bibby, G., et al. 2022. Capturing the RNA castle: exploiting microRNA inhibition for wound healing. FEBS Journal 289 (17): 5137–5151.
Varikuti, S., et al. 2021. MicroRNA-21 deficiency promotes the early Th1 immune response and resistance toward visceral leishmaniasis. The Journal of Immunology 207 (5): 1322–1332.
Kumarswamy, R., et al. 2012. Transforming growth factor-beta-induced endothelial-to-mesenchymal transition is partly mediated by microRNA-21. Arteriosclerosis, Thrombosis, and Vascular Biology 32 (2): 361–369.
Xie, J., et al. 2022. Roles of microRNA-21 in skin wound healing: a comprehensive review. Frontiers in Pharmacology 13: 828627.
Kumarswamy, R., I. Volkmann, and T. Thum. 2011. Regulation and function of miRNA-21 in health and disease. RNA Biology 8 (5): 706–713.
Feng, Y.H., and C.J. Tsao. 2016. Emerging role of microRNA-21 in cancer. Biomedical Reports 5 (4): 395–402.
Melnik, B.C. 2015. MiR-21: an environmental driver of malignant melanoma? Journal of Translational Medicine 13: 202.
Zhou, M., et al. 2019. Overexpression of microRNA-21 inhibits the growth and metastasis of melanoma cells by targeting MKK3. Molecular Medicine Reports 20 (2): 1797–1807.
Long, S., et al. 2018. MiR-21 ameliorates age-associated skin wound healing defects in mice. The Journal of Gene Medicine 20 (6): e3022.
Li, Q., et al. 2019. Human keratinocyte-derived microvesicle miRNA-21 promotes skin wound healing in diabetic rats through facilitating fibroblast function and angiogenesis. International Journal of Biochemistry & Cell Biology 114: 105570.
Lv, Q., et al. 2020. Engineered human adipose stem-cell-derived exosomes loaded with miR-21-5p to promote diabetic cutaneous wound healing. Molecular Pharmaceutics 17 (5): 1723–1733.
Yang, C., et al. 2020. Highly-expressed micoRNA-21 in adipose derived stem cell exosomes can enhance the migration and proliferation of the HaCaT cells by increasing the MMP-9 expression through the PI3K/AKT pathway. Archives of Biochemistry and Biophysics 681: 108259.
Wang, J., et al. 2021. Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. Journal of Nanobiotechnology 19 (1): 202.
Liu, S.C., et al. 2021. Adipose-derived stem cell induced-tissue repair or wound healing is mediated by the concomitant upregulation of miR-21 and miR-29b expression and activation of the AKT signaling pathway. Archives of Biochemistry and Biophysics 705: 108895.
Ma, X., et al. 2011. Loss of the miR-21 allele elevates the expression of its target genes and reduces tumorigenesis. Proceedings of the National Academy of Sciences 108 (25): 10144–10149.
Zhang, Q., et al. 2020. Early-stage bilayer tissue-engineered skin substitute formed by adult skin progenitor cells produces an improved skin structure in vivo. Stem Cell Research & Therapy 11 (1): 407.
Wen, J., et al. 2018. Y-27632 simplifies the isolation procedure of human primary epidermal cells by selectively blocking focal adhesion of dermal cells. Journal of Tissue Engineering and Regenerative Medicine 12 (2): e1251–e1255.
Liu, P., et al. 2022. Exosomes derived from hypoxia-conditioned stem cells of human deciduous exfoliated teeth enhance angiogenesis via the transfer of let-7f-5p and miR-210-3p. Frontiers in Cell and Developmental Biology 10: 879877.
Zhuang, D., et al. 2022. Phenformin suppresses angiogenesis through the regulation of exosomal microRNA-1246 and microRNA-205 levels derived from oral squamous cell carcinoma cells. Frontiers in Oncology 12: 943477.
Li, X.J., et al. 2017. Exosomal microRNA MiR-1246 promotes cell proliferation, invasion and drug resistance by targeting CCNG2 in breast cancer. Cellular Physiology and Biochemistry 44 (5): 1741–1748.
Galiano, R.D., et al. 2004. Quantitative and reproducible murine model of excisional wound healing. Wound Repair and Regeneration 12 (4): 485–492.
Lee, J.G., and M. Heur. 2013. Interleukin-1beta enhances cell migration through AP-1 and NF-kappaB pathway-dependent FGF2 expression in human corneal endothelial cells. Biology of the Cell 105 (4): 175–189.
Tottoli, E.M., et al. 2020. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 12 (8): 735.
Velnar, T., T. Bailey, and V. Smrkolj. 2009. The wound healing process: an overview of the cellular and molecular mechanisms. Journal of International Medical Research 37 (5): 1528–1542.
Sheedy, F.J. 2015. Turning 21: induction of miR-21 as a key switch in the inflammatory response. Frontiers in Immunology 6: 19.
Sonkoly, E., et al. 2007. MicroRNAs: novel regulators involved in the pathogenesis of psoriasis? PLoS ONE 2 (7): e610.
Chen, Y., et al. 2013. HCV-induced miR-21 contributes to evasion of host immune system by targeting MyD88 and IRAK1. PLoS Pathogens 9 (4): e1003248.
Wu, Z., et al. 2012. Inductive microRNA-21 impairs anti-mycobacterial responses by targeting IL-12 and Bcl-2. FEBS Letters 586 (16): 2459–2467.
Das, A., et al. 2014. Engulfment of apoptotic cells by macrophages: a role of microRNA-21 in the resolution of wound inflammation. The Journal of Immunology 192 (3): 1120–1129.
Ando, Y., et al. 2013. Overexpression of microRNA-21 is associated with elevated pro-inflammatory cytokines in dominant-negative TGF-beta receptor type II mouse. Journal of Autoimmunity 41: 111–119.
Guinea-Viniegra, J., et al. 2014. Targeting miR-21 to treat psoriasis. Science Translational Medicine 6 (225): 225re1.
O’Shea, J.J., and P.J. Murray. 2008. Cytokine signaling modules in inflammatory responses. Immunity 28 (4): 477–487.
Xiao, T., et al. 2020. Proinflammatory cytokines regulate epidermal stem cells in wound epithelialization. Stem Cell Research & Therapy 11 (1): 232.
Lee, D.E., N. Ayoub, and D.K. Agrawal. 2016. Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Research & Therapy 7: 37.
Ma, L., et al. 2014. Interleukin-1 beta guides the migration of cortical neurons. Journal of Neuroinflammation 11: 114.
Wang, Z., et al. 2011. Interleukin-lbeta induces migration of rat arterial smooth muscle cells through a mechanism involving increased matrix metalloproteinase-2 activity. Journal of Surgical Research 169 (2): 328–336.
Naldini, A., et al. 2010. Interleukin-1beta regulates the migratory potential of MDAMB231 breast cancer cells through the hypoxia-inducible factor-1alpha. European Journal of Cancer 46 (18): 3400–3408.
White, S.R., et al. 2008. Interleukin-1beta mediates human airway epithelial cell migration via NF-kappaB. American Journal of Physiology. Lung Cellular and Molecular Physiology 295 (6): L1018–L1027.
Oliveira, S.H., et al. 2008. Neutrophil migration induced by IL-1beta depends upon LTB4 released by macrophages and upon TNF-alpha and IL-1beta released by mast cells. Inflammation 31 (1): 36–46.
Yan, B., et al. 2014. IL-1beta and reactive oxygen species differentially regulate neutrophil directional migration and Basal random motility in a zebrafish injury-induced inflammation model. The Journal of Immunology 192 (12): 5998–6008.
Striedinger, K., and E. Scemes. 2008. Interleukin-1beta affects calcium signaling and in vitro cell migration of astrocyte progenitors. Journal of Neuroimmunology 196 (1–2): 116–123.
Liechty, C., et al. 2020. Role of microRNA-21 and its underlying mechanisms in inflammatory responses in diabetic wounds. International Journal of Molecular Sciences 21 (9): 3328.
Madhyastha, R., et al. 2021. MicroRNA 21 elicits a pro-inflammatory response in macrophages, with exosomes functioning as delivery vehicles. Inflammation 44 (4): 1274–1287.
Meng, F., et al. 2007. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 133 (2): 647–658.
Acknowledgements
We gratefully acknowledge Xunwei Wu’s lab members for helpful discussions and technical assistance.
Funding
This work was supported by the General Program of the National Natural Science Foundation of China (82273554, 82073470) and the Shandong Provincial Key R&D Program (ZR2019ZD36) to X.W., and the Key R&D Program of Shandong Province (2018GSF118240) and “Fan 3315 plan” Medical and Health Innovative talents of Ningbo City (2020B-30-G) to J.G.
Author information
Authors and Affiliations
Contributions
Data curation, C.L., Q.Z., and Z.L.; formal analysis, C.L. and X.W.; funding acquisition, J.G. and X.W.; investigation, C.L., Q.Z., Z.L., D.Z., S.W., H.D., J.S., and Y.S.; methodology, Q.Z., Z.L., and X.W.; supervision, J.G., F.W., and X.W.; validation, F.W. and X.W.; writing original draft, C.L.; writing review and editing, X.W. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
The studies involving human participants were reviewed and approved for the use of human tissues collected from discarded hospital specimens without any personal identity information and the protocol (protocol no. GR201711, date February 27, 2017) was approved by the Ethics Committee of the Hospital of Stomatology, Shandong University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. The animal protocol carried out in this study was reviewed and approved by the Ethics Committee of the Hospital of Stomatology, Shandong University (protocol no. GR201720, date February 27, 2017).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, C., Zhang, Q., Liu, Z. et al. miR-21 Expressed by Dermal Fibroblasts Enhances Skin Wound Healing Through the Regulation of Inflammatory Cytokine Expression. Inflammation 47, 572–590 (2024). https://doi.org/10.1007/s10753-023-01930-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-023-01930-2