Abstract
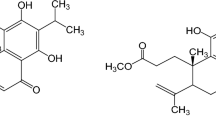
As a lethal infectious disease, tuberculosis (TB) is caused by Mycobacterium tuberculosis (Mtb). Its complex pathophysiological process limits the effectiveness of many clinical treatments. By regulating host cell death, Mtb manipulates macrophages, the first line of defense against invading pathogens, to evade host immunity and promote the spread of bacteria and intracellular inflammatory substances to neighboring cells, resulting in widespread chronic inflammation and persistent lung damage. Autophagy, a metabolic pathway by which cells protect themselves, has been shown to fight intracellular microorganisms, such as Mtb, and they also play a crucial role in regulating cell survival and death. Therefore, host-directed therapy (HDT) based on antimicrobial and anti-inflammatory interventions is a pivotal adjunct to current TB treatment, enhancing anti-TB efficacy. In the present study, we showed that a secondary plant metabolite, ursolic acid (UA), inhibited Mtb-induced pyroptosis and necroptosis of macrophages. In addition, UA induced macrophage autophagy and enhanced intracellular killing of Mtb. To investigate the underlying molecular mechanisms, we explored the signaling pathways associated with autophagy as well as cell death. The results showed that UA could synergistically inhibit the Akt/mTOR and TNF-α/TNFR1 signaling pathways and promote autophagy, thus achieving its regulatory effects on pyroptosis and necroptosis of macrophages. Collectively, UA could be a potential adjuvant drug for host-targeted anti-TB therapy, as it could effectively inhibit pyroptosis and necroptosis of macrophages and counteract the excessive inflammatory response caused by Mtb-infected macrophages via modulating the host immune response, potentially improving clinical outcomes.








Similar content being viewed by others
Availability of Data and Materials
The data used to support the findings of this study are available from the corresponding author upon request.
References
Ravesloot-Chavez, M.M., E. Van Dis, and S.A. Stanley. 2021. The innate immune response to Mycobacterium tuberculosis infection. Annual Review of Immunology 39: 611–637.
Tiberi, S., et al. 2018. Tuberculosis: Progress and advances in development of new drugs, treatment regimens, and host-directed therapies. The Lancet Infectious Diseases 18(7): e183–e198.
Ameisen, J.C. 2002. On the origin, evolution, and nature of programmed cell death: A timeline of four billion years. Cell Death and Differentiation 9(4): 367–393.
Liu, X., and J. Lieberman. 2017. A mechanistic understanding of pyroptosis: The fiery death triggered by invasive infection. Advances in Immunology 135: 81–117.
Weinlich, R., et al. 2017. Necroptosis in development, inflammation and disease. Nature Reviews Molecular Cell Biology 18(2): 127–136.
Frank, D., and J.E. Vince. 2019. Pyroptosis versus necroptosis: Similarities, differences, and crosstalk. Cell Death and Differentiation 26(1): 99–114.
Beckwith, K.S., et al. 2020. Plasma membrane damage causes NLRP3 activation and pyroptosis during Mycobacterium tuberculosis infection. Nature Communications 11(1): 2270.
Li, Y., et al. 2022. Tanshinone IIA alleviates NLRP3 inflammasome-mediated pyroptosis in Mycobacterium tuberculosis-(H37Ra-) infected macrophages by inhibiting endoplasmic reticulum stress. Journal of Ethnopharmacology 282: 114595.
Espert, L., B. Beaumelle, and I. Vergne. 2015. Autophagy in Mycobacterium tuberculosis and HIV infections. Frontiers in Cellular and Infection Microbiology 5: 49.
Castillo, E.F., et al. 2012. Autophagy protects against active tuberculosis by suppressing bacterial burden and inflammation. Proceedings of the National Academy of Sciences of the United States of America 109(46): E3168-E3176.
van der Vaart, M., et al. 2014. The DNA damage-regulated autophagy modulator DRAM1 links mycobacterial recognition via TLR-MYD88 to autophagic defense [corrected]. Cell Host & Microbe 15(6): 753–767.
Zhang, Q., et al. 2017. Antimycobacterial and anti-inflammatory mechanisms of baicalin via induced autophagy in macrophages infected with Mycobacterium tuberculosis. Frontiers in Microbiology 8: 2142.
Mlala, S., et al. 2019. Ursolic acid and its derivatives as bioactive agents. Molecules 24(15): 2751.
Leng, S., et al. 2016. Ursolic acid enhances macrophage autophagy and attenuates atherogenesis. Journal of Lipid Research 57(6): 1006–1016.
Wang, C., et al. 2020. Ursolic acid protects chondrocytes, exhibits anti-inflammatory properties via regulation of the NF-kappaB/NLRP3 inflammasome pathway and ameliorates osteoarthritis. Biomedicine & Pharmacotherapy 130: 110568.
Jimenez-Arellanes, A., et al. 2013. Ursolic and oleanolic acids as antimicrobial and immunomodulatory compounds for tuberculosis treatment. BMC Complementary and Alternative Medicine 13: 258.
Chen, A.Q., et al. 2019. Microglia-derived TNF-alpha mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death & Disease 10(7): 487.
Wang, Y., et al. 2020. TNF-alpha/HMGB1 inflammation signalling pathway regulates pyroptosis during liver failure and acute kidney injury. Cell Proliferation 53(6): e12829.
Rao, Z., et al. 2022. Pyroptosis in inflammatory diseases and cancer. Theranostics 12(9): 4310–4329.
Liang, F., et al. 2020. The advances in pyroptosis initiated by inflammasome in inflammatory and immune diseases. Inflammation Research 69(2): 159–166.
Kesavardhana, S., and T.D. Kanneganti. 2017. Mechanisms governing inflammasome activation, assembly and pyroptosis induction. International Immunology 29(5): 201–210.
Ning, B., et al. 2023. Baicalein suppresses NLRP3 and AIM2 inflammasome-mediated pyroptosis in macrophages infected by Mycobacterium tuberculosis via induced autophagy. Microbiol Spectrum p. e0471122.
Choi, M.E., et al. 2019. Necroptosis: a crucial pathogenic mediator of human disease. JCI Insight 4(15).
Zhang, Y., et al. 2018. Plasma membrane changes during programmed cell deaths. Cell Research 28(1): 9–21.
Lam, A., et al. 2017. Role of apoptosis and autophagy in tuberculosis. American Journal of Physiology. Lung Cellular and Molecular Physiology 313(2): L218–L229.
Weiss, G., and U.E. Schaible. 2015. Macrophage defense mechanisms against intracellular bacteria. Immunological Reviews 264(1): 182–203.
Chai, Q., et al. 2020. New insights into the evasion of host innate immunity by Mycobacterium tuberculosis. Cellular & Molecular Immunology 17(9): 901–913.
Sun, K., et al. 2020. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: A narrative review. Osteoarthritis Cartilage 28(4): 400–409.
Liu, B., et al. 2020. Scoparone improves hepatic inflammation and autophagy in mice with nonalcoholic steatohepatitis by regulating the ROS/P38/Nrf2 axis and PI3K/AKT/mTOR pathway in macrophages. Biomedicine & Pharmacotherapy 125: 109895.
Li, M.Y., et al. 2019. Adrenomedullin alleviates the pyroptosis of Leydig cells by promoting autophagy via the ROS-AMPK-mTOR axis. Cell Death & Disease 10(7): 489.
Meng, Q., et al. 2021. Estrogen prevent atherosclerosis by attenuating endothelial cell pyroptosis via activation of estrogen receptor alpha-mediated autophagy. Journal of Advanced Research 28: 149–164.
Wu, C., et al. 2021. Betulinic acid inhibits pyroptosis in spinal cord injury by augmenting autophagy via the AMPK-mTOR-TFEB signaling pathway. International Journal of Biological Sciences 17(4): 1138–1152.
Xu, C., et al. 2021. TNF-alpha-dependent neuronal necroptosis regulated in Alzheimer’s disease by coordination of RIPK1-p62 complex with autophagic UVRAG. Theranostics 11(19): 9452–9469.
Matsuzawa-Ishimoto, Y., et al. 2017. Autophagy protein ATG16L1 prevents necroptosis in the intestinal epithelium. Journal of Experimental Medicine 214(12): 3687–3705.
Sanz, L., et al. 1999. The interaction of p62 with RIP links the atypical PKCs to NF-kappaB activation. EMBO Journal 18(11): 3044–3053.
Xu, D., C. Zou, and J. Yuan. 2021. Genetic regulation of RIPK1 and necroptosis. Annual Review of Genetics 55: 235–263.
Yao, F., et al. 2022. HDAC11 promotes both NLRP3/caspase-1/GSDMD and caspase-3/GSDME pathways causing pyroptosis via ERG in vascular endothelial cells. Cell Death Discovery 8(1): 112.
Ezquerro, S., et al. 2019. Ghrelin reduces TNF-alpha-induced human hepatocyte apoptosis, autophagy, and pyroptosis: Role in obesity-associated NAFLD. Journal of Clinical Endocrinology and Metabolism 104(1): 21–37.
Hmama, Z., et al. 2015. Immunoevasion and immunosuppression of the macrophage by Mycobacterium tuberculosis. Immunological Reviews 264(1): 220–232.
Lee, K.H., and T.B. Kang. 2019. The molecular links between cell death and inflammasome. Cells 8(9): 1057.
D’Arcy, M.S. 2019. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biology International 43(6): 582–592.
Zhao, J., et al. 2021. Apoptosis, autophagy, NETosis, necroptosis, and pyroptosis mediated programmed cell death as targets for innovative therapy in rheumatoid arthritis. Frontiers in Immunology 12: 809806.
Zhang, R., et al. 2020. Deficiency in the autophagy modulator Dram1 exacerbates pyroptotic cell death of Mycobacteria-infected macrophages. Cell Death & Disease 11(4): 277.
Jouan-Lanhouet, S., et al. 2014. Necroptosis, in vivo detection in experimental disease models. Seminars in Cell & Developmental Biology 35: 2–13.
Riebeling, T., et al. 2021. Primidone blocks RIPK1-driven cell death and inflammation. Cell Death and Differentiation 28(5): 1610–1626.
Wu, W., et al. 2021. TNF-induced necroptosis initiates early autophagy events via RIPK3-dependent AMPK activation, but inhibits late autophagy. Autophagy 17(12): 3992–4009.
Palendira, U., et al. 2002. Lymphocyte recruitment and protective efficacy against pulmonary mycobacterial infection are independent of the route of prior Mycobacterium bovis BCG immunization. Infection and Immunity 70(3): 1410–1416.
Zheng, H., et al. 2008. Genetic basis of virulence attenuation revealed by comparative genomic analysis of Mycobacterium tuberculosis strain H37Ra versus H37Rv. PLoS ONE 3(6): e2375.
Pi, J., et al. 2016. Ursolic acid nanocrystals for dissolution rate and bioavailability enhancement: Influence of different particle size. Current Drug Delivery 13(8): 1358–1366.
Qiu, L., et al. 2019. Ursolic acid nanoparticles for oral delivery prepared by emulsion solvent evaporation method: Characterization, in vitro evaluation of radical scavenging activity and bioavailability. Artif Cells Nanomed Biotechnol 47(1): 610–621.
Panda, S.S., M. Thangaraju, and B.L. Lokeshwar. 2022. Ursolic acid analogs as potential therapeutics for cancer. Molecules 27(24): 8981.
Acknowledgements
The authors thank all the members of the laboratory for their help.
Funding
This study was supported by National Key Research and Development Program of China (2021YFE0200900), National Natural Science Foundation of China (81873069), and Shanghai Municipal Science and Technology Major Project (ZD2021CY001).
Author information
Authors and Affiliations
Contributions
XJ conceived and designed the experiments. JS, YF, FL, and BN performed the experiments. JS and YF analyzed the data. JS and XJ wrote the paper. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shen, J., Fu, Y., Liu, F. et al. Ursolic Acid Promotes Autophagy by Inhibiting Akt/mTOR and TNF-α/TNFR1 Signaling Pathways to Alleviate Pyroptosis and Necroptosis in Mycobacterium tuberculosis-Infected Macrophages. Inflammation 46, 1749–1763 (2023). https://doi.org/10.1007/s10753-023-01839-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-023-01839-w