Abstract
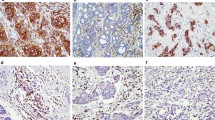
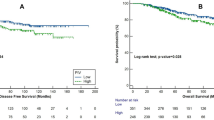
Recent evidence has pointed out that the cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) expression is a poor prognosis factor. However, the implications of CTLA-4 expression on circulating inflammatory mediators are unclear for breast cancer. Tumor biopsies and blood samples were collected from 117 breast cancer patients. Oxidative stress parameters were evaluated in plasma samples by measuring the lipoperoxidation profile and nitric oxide metabolites (NOx). Interleukins 12 (IL-12) and 4 (IL-4) were assessed by ELISA. CTLA-4 expression was determined by immunofluorescence assessed by its labeling in tumor-infiltrating leukocytes (TILs) or breast tumors. Correlations between CTLA-4 expression in breast tumors with TCD4/TCD8 infiltrating lymphocyte and inflammation-related genes were performed using data from TIMER 2.0/TCGA databases (n = 2160). CTLA-4 expression in TILs significantly correlated to triple-negative breast tumors. Patients carrying CTLA-4-positive tumors exhibited lower plasmatic NOx levels, and those expressing CTLA-4 in TILs had reduced levels of IL-12 in plasma. No changes in either IL-4 or lipid peroxidation profiles were detected concerning any CTLA4 status. Compared to the Luminal A ones, oxidative stress parameters and cytokines were observed in patients bearing triple-negative tumors. CTLA-4 expression in all breast cancer subtypes positively correlated to TCD4/TCD8 lymphocyte infiltrates, as well as to the pro-inflammatory genes IL12A, IL4, NFKB1, NFKB2, NOS1, NOS2, and NOS3. CTLA-4 expression in both tumor and TILs can affect the systemic inflammatory status of breast cancer patients, especially antitumor molecules such as IL-12 and NOx that correlate to more aggressive disease.







Similar content being viewed by others
Availability of Data and Material
All data will be available under a reasonable request.
References
Ferlay, J., M. Colombet, I. Soerjomataram, et al. 2019. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. International Journal of Cancer 144: 1941–1953. https://doi.org/10.1002/ijc.31937.
Catsburg, C., A.B. Miller, and T.E. Rohan. 2015. Active cigarette smoking and risk of breast cancer. International Journal of Cancer 136: 2204–2209. https://doi.org/10.1002/ijc.29266.
Panis, C., V.J. Victorino, A.C.S.A. Herrera, et al. 2012. Differential oxidative status and immune characterization of the early and advanced stages of human breast cancer. Breast Cancer Research and Treatment 133: 881–888. https://doi.org/10.1007/s10549-011-1851-1.
Panis, C., V.J. Victorino, A.C.S.A. Herrera, et al. 2015. Can breast tumors affect the oxidative status of the surrounding environment? A comparative analysis among cancerous breast, mammary adjacent tissue, and plasma. Oxidative Medicine and Cellular Longevity 2015: 6429812. https://doi.org/10.1155/2016/6429812.
Wink, D.A., Y. Vodovotz, J. Laval, et al. 2015. The multifaceted roles of nitric oxide in cancer. Carcinogenesis 19: 711–721. https://doi.org/10.1093/carcin/19.5.711.
Hanahan, D., and R.A. Weinberg. 2011. Hallmarks of cancer: The next generation. Cell 144: 646–674. https://doi.org/10.1016/j.cell.2011.02.013.
Pardoll, D.M. 2012. The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer 12: 252–264. https://doi.org/10.1038/nrc3239.
Brunet, J.F., F. Denizot, M.F. Luciani, et al. 1988. A new member of the immunoglobulin superfamily-CTLA-4. Nature 328: 267–270. https://doi.org/10.1038/328267a0.
Mao, H., L. Zhang, Y. Yang, et al. 2010. New insights of CTLA-4 into its biological function in breast cancer. Current Cancer Drug Targets 10: 728–736. https://doi.org/10.2174/156800910793605811.
Erfani, N., M. Razmkhah, and A. Ghaderi. 2010. Circulating soluble CTLA4 (sCTLA4) is elevated in patients with breast cancer. Cancer Investigation 28: 828–832. https://doi.org/10.3109/07357901003630934.
Krummel, M.F., and J.P. Allison. 1996. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. Journal of Experimental Medicine 183: 2533–2540. https://doi.org/10.1084/jem.183.6.2533.
Chambers, C.A., M.S. Kuhns, J.G. Egen, and J.P. Allison. 2001. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annual Review of Immunology 19: 565–594. https://doi.org/10.1146/annurev.immunol.19.1.565.
Burstein, H.J., G. Curigliano, B. Thürlimann, W.P. Weber, P. Poortmans, M.M. Regan, H.J. Senn, E.P. Winer, and M. Gnant. 2021. Panelists of the St Gallen consensus conference. customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Annals of Oncology 32 (10): 1216–1235. https://doi.org/10.1016/j.annonc.2021.06.023.
da Silva, J.C., T.B. Scandolara, R. Kern, H.D.S. Jaques, J. Malanowski, F.M. Alves, D. Rech, G.F. Silveira, and C. Panis. 2022. Occupational exposure to pesticides affects pivotal immunologic anti-tumor responses in breast cancer women from the intermediate risk of recurrence and death. Cancers (Basel). 14 (21): 5199. https://doi.org/10.3390/cancers14215199.
Handala, L., T. Fiore, Y. Rouillé, and F. Helle. 2019. QuantIF: an ImageJ Macro to automatically determine the percentage of infected cells after immunofluorescence. Viruses 11 (2): 165. https://doi.org/10.3390/v11020165.
Panis, C., T.L. Mazzuco, C.Z.F. Costa, et al. 2011. Trypanosoma cruzi: Effect of the absence of 5-lipoxygenase (5-LO)-derived leukotrienes on levels of cytokines, nitric oxide and iNOS expression in cardiac tissue in the acute phase of infection in mice. Experimental Parasitology 127: 58–65. https://doi.org/10.1016/j.exppara.2010.06.030.
Victorino, V.J., F.C. Campos, A.C. Herrera, A.N. Colado Simão, A.L. Cecchini, C. Panis, and R. Cecchini. 2014. Overexpression of HER-2/neu protein attenuates the oxidative systemic profile in women diagnosed with breast cancer. Tumour Biology 35 (4): 3025–3034. https://doi.org/10.1007/s13277-013-1391-x.
Chan, D.V., H.M. Gibson, B.M. Aufiero, A.J. Wilson, M.S. Hafner, Q.S. Mi, and H.K. Wong. 2014. Differential CTLA-4 expression in human CD4+ versus CD8+ T cells is associated with increased NFAT1 and inhibition of CD4+ proliferation. Genes and Immunity 15 (1): 25–32. https://doi.org/10.1038/gene.2013.
Peng, Z., P. Su, Y. Yang, X. Yao, Y. Zhang, F. Jin, and B. Yang. 2020. Identification of CTLA-4 associated with tumor microenvironment and competing interactions in triple negative breast cancer by co-expression network analysis. Journal of Cancer 11 (21): 6365–6375. https://doi.org/10.7150/jca.46301.
Esquivel-Velázquez, M., P. Ostoa-Saloma, M.I. Palacios-Arreola, K.E. Nava-Castro, J.I. Castro, and J. Morales-Montor. 2015. The role of cytokines in breast cancer development and progression. Journal of Interferon and Cytokine Research 35 (1): 1–16. https://doi.org/10.1089/jir.2014.0026.
Kassardjian, A., P.I. Shintaku, N.A. Moatamed. 2018. Expression of immune checkpoint regulators, cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed death-ligand 1 (PD-L1), in female breast carcinomas. PLoS One 13: e0195958. https://doi.org/10.1371/journal.pone.0195958
DeNardo, D.G., L.M. Coussens. 2012. Inflammation and breast cancer. balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Research 9: 212. https://doi.org/10.1186/bcr1746
Frazao, A., M. Messaoudene, N. Nunez, et al. 2019. CD16(+)NKG2A(high) natural killer cells infiltrate breast cancer-draining lymph nodes. Cancer Immunology Research 7: 208–218. https://doi.org/10.1158/2326-6066.CIR-18-0085.
Herrera, A.C.S., V.J. Victorino, F.C. Campos, et al. 2014. Impact of tumor removal on the systemic oxidative profile of patients with breast cancer discloses lipid peroxidation at diagnosis as a putative marker of disease recurrence. Clinical Breast Cancer 14: 451–459. https://doi.org/10.1016/j.clbc.2014.05.002.
Porro, A., C. Chrochemore, F. Cambuli, et al. 2010. Nitric oxide control of MYCN expression and multi drug resistance genes in tumours of neural origin. Current Pharmaceutical Design 16: 431–439. https://doi.org/10.2174/138161210790232112.
Loi, S., N. Sirtaine, F. Piette, et al. 2013. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. Journal of Clinical Oncology. https://doi.org/10.1200/JCO.2011.41.0902.
Stanton, S.E., S. Adams, M.L. Disis. 2016. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncology. https://doi.org/10.1001/jamaoncol.2016.1061.
Herrera, A.C., C. Panis, V.J. Victorino, F.C. Campos, A.N. Colado-Simão, A.L. Cecchini, and R. Cecchini. 2012. Molecular subtype is determinant on inflammatory status and immunological profile from invasive breast cancer patients. Cancer Immunology, Immunotherapy 61 (11): 2193–2201. https://doi.org/10.1007/s00262-012-1283-8.
Hochrein, H., M. O’Keeffe, T. Luft, S. Vandenabeele, R.J. Grumont, E. Maraskovsky, and K. Shortman. 2000. Interleukin (IL)-4 is a major regulatory cytokine governing bioactive IL-12 production by mouse and human dendritic cells. Journal of Experimental Medicine 192 (6): 823–833. https://doi.org/10.1084/jem.192.6.823.
Kriegel, M.A., T. Tretter, N. Blank, et al. 2006. Interleukin-4 supports interleukin-12-induced proliferation and interferon-gamma secretion in human activated lymphoblasts and T helper type 1 cells. Immunology 119 (1): 43–53. https://doi.org/10.1111/j.1365-2567.2006.02404.x.
Kern, R., and C. Panis. 2021. CTLA-4 expression and its clinical significance in breast cancer. Archivum immunolgiae et therapiae experimentalis 69 (1): 16. https://doi.org/10.1007/s00005-021-00618-5.
Zhu, Y., X. Zhu, C. Tang, et al. 2021. Progress and challenges of immunotherapy in triple-negative breast cancer. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer 1876 (2): 188593. https://doi.org/10.1016/j.bbcan.2021.188593. Epub 2021 Jul 17. PMID: 34280474
Mediratta, K., S. El-Sahli, V. D’Costa, et al. 2020. Current progresses and challenges of immunotherapy in triple-negative breast cancer. Cancers (Basel) 12 (12): 3529. https://doi.org/10.3390/cancers12123529.PMID:33256070;PMCID:PMC7761500.
Acknowledgements
The authors are grateful for all lab and hospital personnel, funding agencies, and patients.
Funding
This work was supported by Fundação Araucária, Programa de Pesquisa Para o SUS – PPSUS, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Edital Universal).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, material preparation, data collection, and analysis. Rodrigo Kern and Carolina Panis wrote the first draft of the manuscript, and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
All ethical issues were considered in the study and are reported accordingly in the “Methods” section.
Consent for Publication
All authors gave the consent for paper publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kern, R., da Silva, J.C., Negretti, F. et al. The Expression of CTLA-4 in Breast Tumors and Tumor-Infiltrating Leukocytes Affects Patients’ Systemic Inflammatory Status and Varies According to Their Molecular Subtypes. Inflammation 46, 1639–1652 (2023). https://doi.org/10.1007/s10753-023-01830-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-023-01830-5