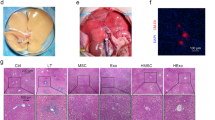
Abstract
Liver injury induced by intestinal ischemia/reperfusion (I/R) is accompanied by the polarization of Kupffer cells, which are specialized macrophages located in the liver. However, the causes of hepatic macrophage polarization after intestinal I/R remain unknown. This study investigated whether gut-derived exosomes contribute to the pathogenesis of liver injury triggered by intestinal I/R in a murine model and explored the underlying mechanisms. Intestinal I/R models were established by temporally clamping the superior mesenteric arteries of mice. Exosomes were isolated from the intestinal tissue of mice that underwent intestinal I/R or sham surgery according to a centrifugation-based protocol. Exosomes were co-cultured with RAW 264.7 macrophages or injected intravenously in mice. Liposomal clodronate was administered intraperitoneally to deplete the macrophages. Macrophage polarization was determined by flow cytometry, immunohistochemistry, and quantitative polymerase chain reaction. Liver injury was assessed by histological morphology and increased serum aspartate aminotransferase and alanine aminotransferase levels. Exosomes from mice intestines subjected to I/R (IR-Exo) promoted macrophage activation in vitro. Intravenous injection of IR-Exo caused hepatic M1 macrophage polarization and led to liver injury in mice. Depleting macrophages ameliorated liver injury caused by intestinal I/R or the injection of IR-Exo. Furthermore, inhibiting exosome release improved intestinal injury, liver function, and survival rates of mice subjected to intestinal I/R. Our study provides evidence that gut-derived exosomes induce liver injury after intestinal I/R by promoting hepatic M1 macrophage polarization. Inhibition of exosome secretion could be a therapeutic target for preventing hepatic impairment after intestinal I/R.







Similar content being viewed by others
DATA AVAILABILITY
The data that support the findings of this study are available on request from the corresponding author.
References
Zhan, Y., Y. Ling, Q. Deng, Y. Qiu, J. Shen, H. Lai, et al. 2022. HMGB1-Mediated Neutrophil Extracellular Trap Formation Exacerbates Intestinal Ischemia/Reperfusion-Induced Acute Lung Injury. Journal of Immunology (Baltimore, Md : 1950) 208: 968–978.
Zhang, Y.N., Z.N. Chang, Z.M. Liu, S.H. Wen, Y.Q. Zhan, H.J. Lai, et al. 2022. Dexmedetomidine Alleviates Gut-Vascular Barrier Damage and Distant Hepatic Injury Following Intestinal Ischemia/Reperfusion Injury in Mice. Anesthesia and Analgesia 134: 419–431.
Hu, J., F. Deng, B. Zhao, Z. Lin, Q. Sun, X. Yang, et al. 2022. Lactobacillus murinus alleviate intestinal ischemia/reperfusion injury through promoting the release of interleukin-10 from M2 macrophages via Toll-like receptor 2 signaling. Microbiome 10: 38.
Gonzalez, L., A. Moeser, and A. Blikslager. 2015. Animal models of ischemia-reperfusion-induced intestinal injury: Progress and promise for translational research. American Journal of Physiology Gastrointestinal and Liver Physiology 308: G63-75.
Horie, Y., R. Wolf, J. Russell, T. Shanley, and D. Granger. 1997. Role of Kupffer cells in gut ischemia/reperfusion-induced hepatic microvascular dysfunction in mice. Hepatology (Baltimore, MD) 26: 1499–1505.
Tacke, F. 2017. Targeting hepatic macrophages to treat liver diseases. Journal of Hepatology 66: 1300–1312.
Wen, S., X. Li, Y. Ling, S. Chen, Q. Deng, L. Yang, et al. 2020. HMGB1-associated necroptosis and Kupffer cells M1 polarization underlies remote liver injury induced by intestinal ischemia/reperfusion in rats. FASEB journal : Official publication of the Federation of American Societies for Experimental Biology 34: 4384–4402.
Mallegol, J., G. Van Niel, C. Lebreton, Y. Lepelletier, C. Candalh, C. Dugave, et al. 2007. T84-intestinal epithelial exosomes bear MHC class II/peptide complexes potentiating antigen presentation by dendritic cells. Gastroenterology 132: 1866–1876.
Kojima, M., J. Gimenes-Junior, T. Chan, B. Eliceiri, A. Baird, T. Costantini, et al. 2018. viaExosomes in postshock mesenteric lymph are key mediators of acute lung injury triggering the macrophage activation Toll-like receptor 4. FASEB journal : Official publication of the Federation of American Societies for Experimental Biology 32: 97–110.
Kojima, M., T. Costantini, B. Eliceiri, T. Chan, A. Baird, and R. Coimbra. 2018. Gut epithelial cell-derived exosomes trigger posttrauma immune dysfunction. The Journal of Trauma and Acute Care Surgery 84: 257–264.
Deng, Z., X. Zhuang, S. Ju, X. Xiang, J. Mu, Y. Liu, et al. 2013. Exosome-like nanoparticles from intestinal mucosal cells carry prostaglandin E2 and suppress activation of liver NKT cells. Journal of Immunology (Baltimore, Md : 1950) 190: 3579–89.
Jiang, L., Y. Shen, D. Guo, D. Yang, J. Liu, X. Fei, et al. 2016. EpCAM-dependent extracellular vesicles from intestinal epithelial cells maintain intestinal tract immune balance. Nature Communications 7: 13045.
Li, Y., Y. Cao, J. Xiao, J. Shang, Q. Tan, F. Ping, et al. 2020. Inhibitor of apoptosis-stimulating protein of p53 inhibits ferroptosis and alleviates intestinal ischemia/reperfusion-induced acute lung injury. Cell Death and Differentiation 27: 2635–2650.
Turnage, R., K. Kadesky, S. Myers, K. Guice, and K. Oldham. 1996. Hepatic hypoperfusion after intestinal reperfusion. Surgery 119: 151–160.
Okada, M., L. Falcão, D. Ferez, J. Martins, P. Errante, F. Rodrigues, et al. 2017. Effect of atenolol pre-treatment in heart damage in a model of intestinal ischemia-reperfusion. Acta Cirúrgica Brasileira 32: 964–972.
Chen, R., Z. Zeng, Y. Zhang, C. Cao, H. Liu, W. Li, et al. 2020. Ischemic postconditioning attenuates acute kidney injury following intestinal ischemia-reperfusion through Nrf2-regulated autophagy, anti-oxidation, and anti-inflammation in mice. FASEB journal : Official publication of the Federation of American Societies for Experimental Biology 34: 8887–8901.
Zhou, J., W. Huang, C. Li, G. Wu, Y. Li, S. Wen, et al. 2012. Intestinal ischemia/reperfusion enhances microglial activation and induces cerebral injury and memory dysfunction in rats. Critical Care Medicine 40: 2438–2448.
Hayase, N., K. Doi, T. Hiruma, R. Matsuura, Y. Hamasaki, E. Noiri, et al. 2019. Recombinant Thrombomodulin on Neutrophil Extracellular Traps in Murine Intestinal Ischemia-Reperfusion. Anesthesiology 131: 866–882.
Fan, X., J. Du, M. Wang, J. Li, B. Yang, Y. Chen, et al. 2019. Irisin Contributes to the Hepatoprotection of Dexmedetomidine during Intestinal Ischemia/Reperfusion. Oxidative Medicine and Cellular Longevity 2019: 7857082.
Zhao, H., F. Zhang, G. Shen, Y. Li, Y. Li, H. Jing, et al. 2010. Sulforaphane protects liver injury induced by intestinal ischemia reperfusion through Nrf2-ARE pathway. World Journal of Gastroenterology 16: 3002–3010.
Han, S., H. Li, M. Kim, V. D’Agati, and H. Lee. 2019. Intestinal Toll-like receptor 9 deficiency leads to Paneth cell hyperplasia and exacerbates kidney, intestine, and liver injury after ischemia/reperfusion injury. Kidney International 95: 859–879.
Horie, Y., R. Wolf, M. Miyasaka, D. Anderson, and D. Granger. 1996. Leukocyte adhesion and hepatic microvascular responses to intestinal ischemia/reperfusion in rats. Gastroenterology 111: 666–673.
Khalyfa, A., C. Zhang, A.A. Khalyfa, G.E. Foster, A.E. Beaudin, J. Andrade, et al. 2016. Effect on Intermittent Hypoxia on Plasma Exosomal Micro RNA Signature and Endothelial Function in Healthy Adults. Sleep 39: 2077–2090.
Minghua, W., G. Zhijian, H. Chahua, L. Qiang, X. Minxuan, W. Luqiao, et al. 2018. Plasma exosomes induced by remote ischaemic preconditioning attenuate myocardial ischaemia/reperfusion injury by transferring miR-24. Cell death & disease 9: 320.
Zhang, L., H. Liu, L. Jia, J. Lyu, Y. Sun, H. Yu, et al. 2019. Exosomes Mediate Hippocampal and Cortical Neuronal Injury Induced by Hepatic Ischemia-Reperfusion Injury through Activating Pyroptosis in Rats. Oxidative medicine and cellular longevity 2019: 3753485.
Chen, X., J. Zhao, Z. Yan, B. Zhou, W. Huang, W. Liu, et al. 2020. Isolation of extracellular vesicles from intestinal tissue in a mouse model of intestinal ischemia/reperfusion injury. BioTechniques 68: 257–262.
Willekens, F., J. Werre, J. Kruijt, B. Roerdinkholder-Stoelwinder, Y. Groenen-Döpp, A. van den Bos, et al. 2005. Liver Kupffer cells rapidly remove red blood cell-derived vesicles from the circulation by scavenger receptors. Blood 105: 2141–2145.
Imai, T., Y. Takahashi, M. Nishikawa, K. Kato, M. Morishita, T. Yamashita, et al. 2015. Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. Journal of extracellular vesicles 4: 26238.
Sica, A., M. Erreni, P. Allavena, and C. Porta. 2015. Macrophage polarization in pathology. Cellular and molecular life sciences : CMLS 72: 4111–4126.
Zhao, Z., L. Zhong, P. Li, K. He, C. Qiu, L. Zhao, et al. 2020. Cholesterol impairs hepatocyte lysosomal function causing M1 polarization of macrophages via exosomal miR-122-5p. Experimental Cell Research 387: 111738.
Liu, X., Q. Pan, H. Cao, F. Xin, Z. Zhao, R. Yang, et al. 2020. Lipotoxic hepatocyte-derived exosomal MicroRNA 192–5p Activates macrophages through rictor/Akt/forkhead box transcription factor O1 signaling in nonalcoholic fatty liver disease. Hepatology (Baltimore, MD) 72: 454–469.
Xiaoming, A., J. Wenbo, W. Jinyi, W. Bin, H. Chunyang, C. Qi, et al. 2020. Macrophage regnase-1 deletion deteriorates liver ischemia/reperfusion injury through regulation of macrophage polarization. Frontiers in Physiology 11: 582347.
Catalano, M., and L. O’Driscoll. 2020. Inhibiting extracellular vesicles formation and release: A review of EV inhibitors. Journal of extracellular vesicles 9: 1703244.
Essandoh, K., L. Yang, X. Wang, W. Huang, D. Qin, J. Hao, et al. 2015. Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochimica et Biophysica Acta 1852: 2362–2371.
Chen, Y., H. Sun, Y. Bai, and F. Zhi. 2019. Gut dysbiosis-derived exosomes trigger hepatic steatosis by transiting HMGB1 from intestinal to liver in mice. Biochemical and Biophysical Research Communications 509: 767–772.
ACKNOWLEDGEMENTS
We thank all the funds mentioned above for their support.
Funding
This work was supported by grants from National Natural Science Foundation, Beijing, China (81871609 to Cai Li, 81671955 to Ke-Xuan Liu), Key Program of National Natural Science Foundation, Beijing, China (81730058 to Ke-Xuan Liu).
Author information
Authors and Affiliations
Contributions
Study conception/design: Jin Zhao, Ke-Xuan Liu, Cai Li. Conduct of experiments: Jin Zhao, Xiao-Dong Chen. Data analysis: Jin Zhao, Zhengzheng Yan, Cai Li. Drafting of paper: Jin Zhao. Editing/revision of paper: Cai Li, Ke-Xuan Liu. Reading and approval of the final version of the paper: all authors
Corresponding authors
Ethics declarations
Ethics Approval
This study was approved by the Institutional Animal Care and Use Committee of Southern Medical University (Application number: NFYY-2018–0013).
Consent for Publication
All authors have agreed for the publication of this paper.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, J., Chen, XD., Yan, ZZ. et al. Gut-Derived Exosomes Induce Liver Injury After Intestinal Ischemia/Reperfusion by Promoting Hepatic Macrophage Polarization. Inflammation 45, 2325–2338 (2022). https://doi.org/10.1007/s10753-022-01695-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-022-01695-0