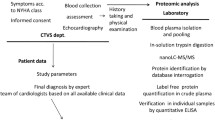
Abstract
Rheumatic heart disease (RHD) is a major cause of cardiovascular morbidity and mortality in low- and middle-income countries, where living conditions promote spread of group A β-haemolytic streptococcus. Autoimmune reactions due to molecular mimicry of bacterial epitopes by host proteins cause acute rheumatic fever (ARF) and subsequent disease progression to RHD. Despite knowledge of the factors that predispose to ARF and RHD, determinants of the progression to valvular damage and the molecular events involved remain incompletely characterised. This review focuses on altered protein expression in heart valves, myocardial tissue and plasma of patients with RHD and pathogenic consequences on RHD. Proteins mainly involved in structural organization of the valve matrix, blood homeostasis and immune response were altered due to RHD pathogenesis. Study of secreted forms of these proteins may aid the development of non-invasive biomarkers for early diagnosis and monitoring outcomes in RHD. Valve replacement surgery, the single evidence-based strategy to improve outcomes in severe RHD, is costly, largely unavailable in low- and middle-income countries (LMIC) and requires specialised facilities. When diagnosed early, penicillin prophylaxis may be used to delay progression to severe valvular damage. Echocardiography and cardiovascular magnetic resonance and the standard imaging tools recommended to confirm early diagnosis remain largely unavailable and inaccessible in most LMIC and both require expensive equipment and highly skilled persons for manipulation as well as interpretation of results. Changes in protein expression in heart valves and myocardium are associated with progressive valvular deformation in RHD. Understanding these protein changes should shed more light on the mechanisms of pathogenicity, while secreted forms of these proteins may provide leads towards a biomarker for non-invasive early detection of RHD.


Similar content being viewed by others
References
Carapetis JR, Beaton A, Cunningham MW, Guilherme L, Karthikeyan G, Mayosi BM, et al. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Prim [Internet]. 2016 Jan 14;2:15084. Available from: https://doi.org/10.1038/nrdp.2015.84
Marijon E, Mirabel M, Celermajer DS, Jouven X (2012) Rheumatic heart disease. Lancet [Internet] 379(9819):953–964. https://doi.org/10.1016/S0140-6736(11)61171-9
Guilherme L, Kalil J. Rheumatic heart disease: molecules involved in valve tissue inflammation leading to the autoimmune process and anti-S. pyogenes vaccine. Front Immunol [Internet]. 2013;4(October):1–6. Available from: http://journal.frontiersin.org/article/10.3389/fimmu.2013.00352/abstract
Carapetis JR, Steer AC, Mulholland EK, Weber M (2005) The global burden of group A streptococcal diseases. Lancet Infect Dis 5(11):685–694
Lee JL, Naguwa SM, Cheema GS, Gershwin ME (2009) Acute rheumatic fever and its consequences: a persistent threat to developing nations in the 21st century. Autoimmun Rev [Internet] 9(2):117–123. https://doi.org/10.1016/j.autrev.2009.04.002
Carapetis JR (2015) The stark reality of rheumatic heart disease. Eur Heart J 36(18):1070–1073
Guilherme L, Köhler KF, Pommerantzeff P, Spina G, Kalil J (2013) Rheumatic heart disease: key points on valve lesions development. J Clin Exp Cardiol S 3:2
Mukherjee S, Jagadeeshaprasad MG, Banerjee T, Ghosh SK, Biswas M, Dutta S, et al. Proteomic analysis of human plasma in chronic rheumatic mitral stenosis reveals proteins involved in the complement and coagulation cascade. Clin Proteomics [Internet]. 2014;11(1):35. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4193131&tool=pmcentrez&rendertype=abstract
Zühlke LJ, Steer AC (2013) Estimates of the global burden of rheumatic heart disease. Glob Heart [Internet] 8(3):189–195. https://doi.org/10.1016/j.gheart.2013.08.008
Sliwa K, Carrington M, Mayosi BM, Zigiriadis E, Mvungi R, Stewart S (2010) Incidence and characteristics of newly diagnosed rheumatic heart disease in urban African adults: insights from the heart of Soweto study. Eur Heart J 31(6):719–727
Zühlke LJ, Engel ME, Watkins D, Mayosi BM (2015) Incidence, prevalence and outcome of rheumatic heart disease in South Africa: a systematic review of contemporary studies. Int J Cardiol [Internet]. 199(April 2014):375–383. https://doi.org/10.1016/j.ijcard.2015.06.145
Engel ME, Haileamlak A, Zühlke L, Lemmer CE, Nkepu S, van de Wall M, Daniel W, Shung King M, Mayosi BM (2015) Prevalence of rheumatic heart disease in 4720 asymptomatic scholars from South Africa and Ethiopia. Heart [Internet] 101(17):1389–1394. https://doi.org/10.1136/heartjnl-2015-307444
Beaton A, Okello E, Lwabi P, Mondo C, McCarter R, Sable C (2012) Echocardiography screening for rheumatic heart disease in Ugandan schoolchildren. Circulation. 125(25):3127–3132
Damasceno A, Mayosi BM, Sani M, Ogah OS, Mondo C, Ojji D, Dzudie A, Kouam CK, Suliman A, Schrueder N, Yonga G, Ba SA, Maru F, Alemayehu B, Edwards C, Davison BA, Cotter G, Sliwa K (2012) The causes, treatment, and outcome of acute heart failure in 1006 Africans from 9 countries: results of the sub-Saharan Africa survey of heart failure. Arch Intern Med 172(18):1386–1394
Otto H, Saether SG, Banteyrga L, Haugen BO, Skjaerpe T (2011) High prevalence of subclinical rheumatic heart disease in pregnant women in a developing country: an echocardiographic study. Echocardiography [Internet] 28(10):1049–1053. https://doi.org/10.1111/j.1540-8175.2011.01520.x
Zuhlke LJ, Beaton A, Engel ME, Hugo-Hamman CT, Karthikeyan G, Katzenellenbogen JM, et al. Group A Streptococcus, acute rheumatic fever and rheumatic heart disease: epidemiology and clinical considerations. Curr Treat Options Cardiovasc Med. 2017;19(2)
Guzman-Cottrill JAU, Jaggi P, Shulman ST (2004) Acute rheumatic fever: clinical aspects and insights into pathogenesis and prevention. Clin Appl Immunol Rev 4(4):263–276
Remenyi B, Wilson N, Steer A, Ferreira B, Kado J, Kumar K, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease--an evidence-based guideline. Nat Rev Cardiol [Internet]. 2012;9(5):297–309. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22371105
Sika-paotonu D, Beaton A, Raghu A, Steer A, Carapetis J. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Prim [Internet]. 2016;15085. Available from: http://www.nature.com/articles/nrdp201585
Cunningham MW (2000) Pathogenesis of group a streptococcal infections. Clin Microbiol Rev 13(3):470–511
Mccarthy KP, Ring L, Rana BS (2010) Anatomy of the mitral valve: understanding the mitral valve complex in mitral regurgitation. Eur J Echocardiogr 11:3–9
Lee JKT, Franzone A, Lanz J, Siontis GCM, Stortecky S, Gräni C, Roost E, Windecker S, Pilgrim T Early detection of subclinical myocardial damage in chronic aortic regurgitation and strategies for timely treatment of asymptomatic patients. Circulation [Internet] 2018;137(2):184–96. Available from: http://circ.ahajournals.org/lookup/doi/10.1161/CIRCULATIONAHA.117.029858
Carabello BA (2005) Modern management of mitral stenosis. Circulation. 112(3):432–437
Gewitz MH, Baltimore RS, Tani LY, Sable CA, Shulman ST, Carapetis J, Remenyi B, Taubert KA, Bolger AF, Beerman L, Mayosi BM, Beaton A, Pandian NG, Kaplan EL, American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young (2015) Revision of the Jones criteria for the diagnosis of acute rheumatic fever in the era of Doppler echocardiography a scientific statement from the American heart association. Circulation. 131(20):1806–1818
Remenyi B, Elguindy A, Smith SC, Yacoub M, Holmes DR (2016) Valvular aspects of rheumatic heart disease. Lancet. 387(10025):1335–1346
Ntusi NA, Piechnik SK, Francis JM, Ferreira VM, Rai AB, Matthews PM, et al. Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis – a clinical study using myocardial T1-mapping and extracellular volume quantification. J Cardiovasc Magn Reson [Internet] 2014;16(21):1–12. Available from: http://jcmr-online.com/content/16/1/21
Moosa S, Ntusi NAB, Town C, Africa S, Africa S, Hospital GS et al (2016) Role of cardiovascular magnetic resonance in the evaluation of cardiomyopathy. SA J Radiol:1–10
Butcovan D, Arsenescu C, Georgescu GI, Borza C, Tinica G, Sandica E, et al. Morphological evaluation of the surgically removed aortic and mitral valves. Rev Med Chir Soc Med Nat Iasi. 2004;108(Romania PT-Journal Article LG-English OVID MEDLINE UP 20081216):66–73
Boudoulas KD, Borer JS, Boudoulas H. Etiology of valvular heart disease in the 21st century. Cardiology [Internet] 2013;126(3):139–52. Available from: http://www.karger.com?doi=10.1159/000354221
Cunningham MW (2012) Streptococcus and rheumatic fever. Curr Opin Rheumatol 24(4):408–416
McNamara C, Zinkernagel AS, Macheboeuf P, Cunningham MW, Nizet V, Ghosh P. Coiled-coil irregularities and instabilities in group A Streptococcus M1 are required for virulence. Science 319: 5868:1405-1408. Available from: https://doi.org/10.1126/science.1154470
Guilherme L, Kalil J (2010) Rheumatic fever and rheumatic heart disease: cellular mechanisms leading autoimmune reactivity and disease. J Clin Immunol 30(1):17–23
Sridhar S, CPharm DH, Gee TW, Hua J, Butcher J. Monocytes and macrophages in heart valves: uninvited guests or critical performers? Autex Res J [Internet]. 2009;9(3):74–81. Available from: https://doi.org/10.1016/j.cobme.2018.02.003, 2018
Salhiyyah K, Yacoub MH, Chester AH (2011) Cellular mechanisms in mitral valve disease. J Cardiovasc Transl Res 4(6):702–709
Dweck MR, Boon NA, Newby DE (2012) Calcific aortic stenosis: a disease of the valve and the myocardium. J Am Coll Cardiol [Internet]. 60(19):1854–1863. https://doi.org/10.1016/j.jacc.2012.02.093
Zhang W, Mondo C, Okello E, Musoke C, Kakande B, Nyakoojo W, Kayima J, Freers J Presenting features of newly diagnosed rheumatic heart disease patients in Mulago Hospital: a pilot study. Cardiovasc J Afr [Internet] 2013;24(2):28–33. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3734881&tool=pmcentrez&rendertype=abstract
de CO M, Santos KS, Ferreira FM, Teixeira PC, PMA P, CMA B et al (2014) Distinct mitral valve proteomic profiles in rheumatic heart disease and Myxomatous degeneration. Clin Med Insights Cardiol 8:79–86
de CO M, Demarchi L, Ferreira FM, PMA P, Brandao C, Sampaio RO et al (2017) Rheumatic heart disease and myxomatous degeneration: differences and similarities of valve damage resulting from autoimmune reactions and matrix disorganization. PLoS One 12(1):1–12
Fae KC, Diefenbach da Silva D, AMB B, Tanaka AC, PMA P, Kiss MH et al (2008) PDIA3, HSPA5 and vimentin, proteins identified by 2-DE in the valvular tissue, are the target antigens of peripheral and heart infiltrating T cells from chronic rheumatic heart disease patients. J Autoimmun 31(2):136–141
Saikia UN, Kumar RM, Pandian VKGRP, Gupta S, Dhaliwal RS, Talwar KK (2012) Adhesion molecule expression and ventricular remodeling in chronic rheumatic heart disease: a cause or effect in the disease progression - a pilot study. Cardiovasc Pathol [Internet]. 21(2):83–88. https://doi.org/10.1016/j.carpath.2011.01.005
Kong P, Christia P, Frangogiannis NG (2014) The pathogenesis of cardiac fibrosis:549–574
Kehat I, Molkentin J (2010) Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation. 122:2727–2735
Solaro RJ (2010) Sarcomere control mechanisms and the dynamics of the cardiac cycle. J Biomed Biotechnol 2010:1–8
Gaasch WH, Meyer TE. Left ventricular response to mitral regurgitation implications for management. Vol. 118, Circulation. 2008. p. 2298–303
Enriquez-sarano M, Basmadjian A, Rossi A, Bailey KR, Seward JB, Tajik AJ (1999) Progression of mitral regurgitation a prospective Doppler echocardiographic study. J Am Coll Cardiol [Internet] 34(4):1137–1144. https://doi.org/10.1016/S0735-1097(99)00313-7
Maganti K, Rigolin VH, Enriquz S, Bonow RO. Valvular heart disease: diagnosis and management. Mayo Clin Proc @BULLET May [Internet] 2010;85(5):483–500. Available from: www.mayoclinicproceedings.com
Banerjee T, Mukherjee S, Ghosh S, Biswas M, Dutta S, Pattari S, et al. Clinical significance of markers of collagen metabolism in rheumatic mitral valve disease. PLoS One. 2014;9(3)
Banerjee T, Mukherjee S, Biswas M, Dutta S, Chatterjee S, Ghosh S, Pattari S, Nanda NC, Bandyopadhyay A Circulating carboxy-terminal propeptide of type I procollagen is increased in rheumatic heart disease. Int J Cardiol [Internet] 2012;156(1):117–9. Available from: https://doi.org/10.1016/j.ijcard.2012.01.026
Aoki A, Ashizawa T, Ebata A, Nasu Y, Fujii T (2014) Group A Streptococcus pharyngitis outbreak among university students in a judo club. J Infect Chemother [Internet] 20(3):190–193. https://doi.org/10.1016/j.jiac.2013.10.004
Kaplan EL (2005) Pathogenesis of acute rheumatic fever and rheumatic heart disease: evasive after half a century of clinical, epidemiological, and laboratory investigation. Heart. 91(1):3–4
Guilherme L, Kalil J, Cunningham MM (2006) Molecular mimicry in the autoimmune pathogenesis of rheumatic heart disease. Autoimmunity. 39(1):31–39
Guilherme L, Kohler K, Kalil J (2011) Rheumatic heart disease: mediation by complex immune events. Adv Clin Chem 53(11):31–50
Guilherme L, Weidebach W, Kiss M, Snitcowsky R, Kalil J (1991) Association of human leukocyte class II antigens with rheumatic fever or rheumatic heart disease in a Brazilian population. Circulation. 83(6):1995–1998
Gomaa MH, Ali SS, Fattouh AM, Hamza HS (1624) MBL2 gene polymorphism rs1800450 and rheumatic fever with and without rheumatic heart disease: an Egyptian pilot study. Paediatr Rheumatol 2018:1–7
Schafranski MD, Stier A, Nisihara R, Messias-Reason IJT (2004) Significantly increased levels of mannose-binding lectin (MBL) in rheumatic heart disease: a beneficial role for MBL deficiency. Clin Exp Immunol 138(3):521–525
Ramasawmy R, Spina GS, Fae KC, Pereira AC, Nisihara R, Reason IJM et al (2008) Association of mannose-binding lectin gene polymorphism but not of mannose-binding serine protease 2 with chronic severe aortic regurgitation of rheumatic etiology. Clin Vaccine Immunol 15(6):932–936
dos SJS C, ABW B, Beltrame MH, Nisihara RM, Schafranski MD, de Messias-Reason IJ (2014) Association of MASP2 polymorphisms and protein levels with rheumatic fever and rheumatic heart disease. Hum Immunol [Internet] 75(12):1197–1202. https://doi.org/10.1016/j.humimm.2014.10.003
Ellis NMJ, Li Y, Hildebrand W, Fischetti VA, Cunningham MW. T cell mimicry and epitope specificity of cross-reactive T cell clones from rheumatic heart disease. J Immunol [Internet] 2005;175(8):5448–56. Available from: http://www.jimmunol.org/cgi/doi/10.4049/jimmunol.175.8.5448
Tandon R, Sharma M, Chandrashekhar Y, Kotb M, Yacoub MH, Narula J. Revisiting the pathogenesis of rheumatic fever and carditis. Nat Rev Cardiol. 2013;10(3)
Dinkla K, Rohde M, Jansen WTM, Carapetis JR, Chhatwal GS, Talay SR (2003) Streptococcus pyogenes recruits collagen via surface-bound fibronectin: a novel colonization and immune evasion mechanism. Mol Microbiol 47(3):861–869
Dinkla K, Talay SR, Mörgelin M, Graham RMA, Rohde M, Nitsche-Schmitz DP et al (2009) Crucial role of the CB3-region of collagen IV in PARF-induced acute rheumatic fever. PLoS One 4(3):1–8
Terao Y. The virulence factors and pathogenic mechanisms of Streptococcus pyogenes. J Oral Biosci [Internet] 2012;54(2):96–100. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1349007912000497
Freedman BR, Bade ND, Riggin CN, Zhang S, Haines PG, Ong KL, Janmey PA (2015) The ( dys ) functional extracellular matrix. Biochim Biophys Acta [Internet] 1853(11):3153–3164. https://doi.org/10.1016/j.bbamcr.2015.04.015
Guilherme L, Cury P, Demarchi LMF, Lopez AP, Oshiro SE, Aliotti S, et al. Rheumatic heart disease proinflammatory cytokines play a role in the progression and 2004;165(5):1583–91
Guilherme L, Faé KC, Oshiro SE, Tanaka AC (2005) Rheumatic fever how S. pyogenes–primed peripheral. T cells 140:132–140
Roberts S, Kosanke S, Dunn ST, Jankelow D, Duran CMG, Cunningham MW. Pathogenic mechanisms in rheumatic carditis: focus on valvular endothelium. J Infect Dis [Internet] 2001;183(3):507–11. http://jid.oxfordjournals.org/content/183/3/507.abstract
Guilherme L, Köhler KF, Kalil J. Rheumatic heart disease: genes, inflammation and autoimmunity. Rheumatol Curr Res [Internet] 2012;S4(01):1–5. Available from: https://www.omicsonline.org/rheumatic-heart-disease-genes-inflammation-and-autoimmunity-2161-1149.S4-001.php?aid=6690
Fae K, Kalil J, Toubert A, Guilherme L (2004) Heart infiltrating T cell clones from a rheumatic heart disease patient display a common TCR usage and a degenerate antigen recognition pattern. Mol Immunol 40(14–15):1129–1135
Guilherme L, Kalil J (2007) Rheumatic fever: from innate to acquired immune response. Ann N Y Acad Sci 1107:426–433
Bas HD, Baser K, Yaniz E, Bolayir HA, Yaman B, Unlu S et al (2014) A shift in the balance of regulatory T and T helper 17 cells in rheumatic heart disease. J Investig Med 62(January):1–6
Mukhopadhyay S, Varma S, Kumar HNM, Yusaf J, Goyal M, Mehta V et al (2016) Circulating level of regulatory T cells in rheumatic heart disease: an observational study. Indian Heart J 8:2–8
Bilik MZ, Kaplan I, Polat N, Akil MA, Akyuz A, Acet H et al (2016) Serum levels of IL-17 and IL-23 in patients with rheumatic mitral stenosis. Medicine (Baltimore) 95(18):1–5
Polat N, Yildiz A, Yuksel M, Bilik MZ, Aydin M, Acet H, et al. Association of neutrophil–lymphocyte ratio with the presence and severity of rheumatic mitral valve stenosis. Clin Appl Thromb. 2014;
Li W, Zeng Z, Gui C, Zheng H, Huang W, Wei H, et al. Proteomic analysis of mitral valve in Lewis rat with acute rheumatic heart disease. Int J Clin Exp Pathol [Internet]. 2015;8(11):14151–60. Available from: internal-pdf://245.162.168.204/Li-2015-Proteomic analysis of mitral valve in.pdf%0Ahttp://www.ncbi.nlm.nih.gov/pubmed/26823728http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4713514/pdf/ijcep0008-14151.pdf
Mayr M, Zhang J, Greene AS, Gutterman D, Perloff J, Ping P (2006) Proteomics-based development of biomarkers in cardiovascular disease: mechanistic, clinical, and therapeutic insights. Mol Cell Proteomics 5(10):1853–1864
Challa AA, Stefanovic B (2011) A novel role of vimentin filaments: binding and stabilization of collagen mRNAs. Mol Cell Biol [Internet] 31(18):3773–3789. https://doi.org/10.1128/MCB.05263-11
Bella J. Collagen structure: new tricks from a very old dog. 2016;4:1001–25
Seiffert D. Constitutive and regulated expression of vitronectin. Histol hostopathology. 1991;12(1 997):787–97
Ringer P, Colo G, Fässler R, Grashoff C. Sensing the mechano-chemical properties of the extracellular matrix. Matrix Biol [Internet]. 2017;64:6–16. Available from: https://doi.org/10.1016/j.matbio.2017.03.004, 2017
Lu Q, Sun Y, Duan Y, Li B, Xia J, Yu S et al (2018) Comprehensive microRNA profiling reveals potential augmentation of the IL1 pathway in rheumatic heart valve disease. BMC Cardiovasc Disord 18(53):1–12
Jiang X, Zhang J, Huang Y (2015) Tetraspanins in cell migration. Cell Adhes Migr 9(July):406–415
Powner D, Kopp PM, Monkley SJ, Critchley DR, Berditchevski F. Tetraspanin CD9 in cell migration. Biochem Soc Trans [Internet] 2011;39(2):563–7. Available from: http://biochemsoctrans.org/lookup/doi/10.1042/BST0390563
Wieten L, Van Der Zee R, Spiering R, Wagenaar-Hilbers J, Van Kooten P, Broere F et al (2010) A novel heat-shock protein coinducer boosts stress protein Hsp70 to activate T cell regulation of inflammation in autoimmune arthritis. Arthritis Rheum 62(4):1026–1035
Zheng D, Xu L, Sun L, Feng Q, Wang Z, Shao G et al (2014) Comparison of the ventricle muscle proteome between patients with rheumatic heart disease and controls with mitral valve prolapse: HSP 60 may be a specific protein in RHD. Biomed Res Int 2014:1–9
Tontsch D, Pankuweit S, Marburg P. Autoantibodies in the sera of patients with rheumatic heart disease: characterization of myocardial antigens by two-dimensional immunoblotting and N-terminal sequence analysis 2000;270–4
Yuan C, Solaro RJ. Myofilament proteins: from cardiac disorders to proteomic changes. Vol. 2, Proteomics - Clinical Applications. 2008. p. 788–99
Gupta S, Knowlton AA. HSP60, Bax, apoptosis and the heart. Vol. 9, Journal of Cellular and Molecular Medicine. 2005. p. 51–8
Kumar RK, Tandon R (2013) Rheumatic fever & rheumatic heart disease: the last 50 years. Indian J Med Res 137(4):643–658
Tian J, Guo X, Liu XM, Liu L, Weng QF, Dong SJ, Knowlton AA, Yuan WJ, Lin L (2013) Extracellular HSP60 induces inflammation through activating and up-regulating TLRs in cardiomyocytes. Cardiovasc Res 98(3):391–401
Wang Y, Chen L, Hagiwara N, Knowlton AA (2010) Regulation of heat shock protein 60 and 72 expression in the failing heart. J Mol Cell Cardiol [Internet] 48(2):360–366. https://doi.org/10.1016/j.yjmcc.2009.11.009
Monreal G, Nicholson LM, Han B, Joshi MS, Phillips AB, Wold LE, Bauer JA, Gerhardt MA (2008) Cytoskeletal remodeling of desmin is a more accurate measure of cardiac dysfunction than fibrosis or myocyte hypertrophy. Life Sci [Internet] 83(23–24):786–794. https://doi.org/10.1016/j.lfs.2008.09.026
Agnetti G, Halperin VL, Kirk JA, Chakir K, Guo Y, Lund L, Nicolini F, Gherli T, Guarnieri C, Caldarera CM, Tomaselli GF, Kass DA, van Eyk JE (2014) Desmin modifications associate with amyloid-like oligomers deposition in heart failure. Cardiovasc Res 102(1):24–34
Rainer PP, Dong P, Sorge M, Fert-bober J, Holewinski RJ, Wang Y et al (2018) Desmin phosphorylation triggers preamyloid oligomers formation and myocyte dysfunction in acquired heart failure. Circ Res 122(10):75–83
Gunning P, Weinberger R, Jeffery P. Actin and tropomyosin isoforms in morphogenesis. 1997;311–315
Kyriakides TR, Bornstein P (2003) Matricellular proteins as modulators of wound healing and the foreign body response. Thromb Haemost 90(6):986–992
Gao G, Xuan C, Yang Q, Liu X, Liu Z, He G. Identification of altered plasma proteins by proteomic study in valvular heart diseases and the potential clinical significance. 2013;8(8)
de Messias IJ, Cavalcanti E, Radominski SC (1995) Increased frequency of the C4A*6 rare allele in rheumatic heart disease. Scand J Rheumatol 24(3):164–168
Wu X, Yue Q, Jia W, Zhang J, Ouyang H, Xin D, et al. A novel approach for characterizing variations in serum peptides in rheumatic heart disease. 2017;(March):365–72
Schwochau GB, Nath KA, Rosenberg ME (1998) Clusterin protects against oxidative stress in vitro through aggregative and nonaggregative properties. Kidney Int 53(6):1647–1653
Zhang H, Kim JK, Edwards CA, Xu Z, Taichman R, Wang CY (2005) Clusterin inhibits apoptosis by interacting with activated Bax. Nat Cell Biol 7(9):909–915
Wen Y, Zeng Z, Gui C, Li L, Li W (2015) Changes in the expression of Th17 cell-associated cytokines in the development of rheumatic heart disease. Cardiovasc Pathol [Internet] 24(6):382–387. https://doi.org/10.1016/j.carpath.2015.07.006
Alyan O, Metin F, Kacmaz F, Ozdemir O, Maden O, Topaloglu S, Demir AD, Karahan Z, Karadede A, Ilkay E (2009) High levels of high sensitivity C-reactive protein predict the progression of chronic rheumatic mitral stenosis. J ournal Thrombolysis 28:63–69
Stastny J, Fosslien E, Robertson AL (1986) Human aortic intima protein composition during initial stages of atherogenesis. Atherosclerosis. 60(2):131–139
Putri R, Suwarniaty R, Fitri L, Nugroho S, Rahman M. Prominently increased of mannose binding lectin (MBL) and myeloperoxidase (MPO) levels in severe valve regurgitation and heart failure of rheumatic heart disease. J Trop Life Sci [Internet] 2017;7(2):108–14. Available from: http://jtrolis.ub.ac.id/index.php/jtrolis/article/view/615
Cagli K, Basar N, Cagli K, Armutcu F, Aylak F, Yalcinkaya A, Erden G, Kadirogullari E (2010) Association of serum fetuin-A with valvular calcium concentration in rheumatic mitral valve disease. J Heart Valve Dis 19(5):636–643
Lopez B, Gonzalez A, Ravassa S, Beaumont J, Moreno MU, San Jose G et al (2015) Circulating biomarkers of myocardial fibrosis: the need for a reappraisal. J Am Coll Cardiol 65(22):2449–2456
Sarkar S, Rastogi M, Chaudhary P, Kumar R, Arora P, Sagar V et al (2017) Association of rheumatic fever & rheumatic heart disease with plausible early & late-stage disease markers. Indian J Med Res 145(June):758–766
Meel R, Nethononda R, Libhaber E, Dix-Peek T, Peters F, Essop R (2018) Assessment of myocardial fibrosis by late gadolinium enhancement imaging and biomarkers of collagen metabolism in chronic rheumatic mitral regurgitation. Cardiovasc J Afr 29(October 2014):1–5
Acknowledgments
We are grateful to Dr. Ivana Parker for useful discussion.
Funding
ENL is funded by the National Research Foundation (NRF) of South Africa (DST-NRF) free standing innovation postdoctoral fellowship and an early career development fellowship from the Carnegie Corporation of New York to the University of Cape Town. SS thanks the NRF Blue Skies for support. JMB thanks the NRF for a South African Research Chair. NNAB gratefully acknowledges support from the NRF, SA-MRC and the Ernst and Lily Hausmann Trust.
Author information
Authors and Affiliations
Contributions
ENL and NNAB contributed to conceptualisation, ENL and NNAB wrote the first draft, NNAB, JMB and SS supervised the work and all the authors contributed to review and editing and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lumngwena, E.N., Skatulla, S., Blackburn, J.M. et al. Mechanistic implications of altered protein expression in rheumatic heart disease. Heart Fail Rev 27, 357–368 (2022). https://doi.org/10.1007/s10741-020-09993-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-020-09993-1