Summary
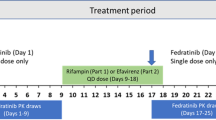
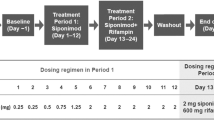
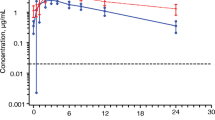
To evaluate the potential drug-drug interaction (DDI), safety and tolerability of fuzuloparib co-administered with a moderate CYP3A inducer efavirenz in healthy male subjects. Eighteen healthy male subjects were enrolled in a single-center, single-arm, open-label, fixed-sequence study. Fuzuloparib was administered as a single oral 50 mg under a fasting state on day 1, efavirenz (600 mg once daily) was given on days 4–17 before bed time, concomitantly with fuzuloparib on day 18, and for the follow-up 3 additional days (days 19–20). Pharmacokinetic sampling was performed following each fuzuloparib dose. Safety and tolerability were assessed during the whole process via clinical laboratory tests. Ratios of least-squares means (GMRs) and 90% geometric confidence interval (90% CI) of maximum plasma concentration (Cmax), the area under the curve of plasma concentration-time from zero to the last measurable concentration (AUC0 − t) and the area under the curve of blood concentration from zero to infinity (AUC0−∞) for fuzuloparib combined with efavirenz to fuzuloparib alone were 0.473 (0.394, 0.568), 0.220 (0.185, 0.263) and 0.221 (0.185, 0.263), respectively. Co-administration with efavirenz led to 53% and 78% decreases in fuzuloparib Cmax and AUC0−∞. All 18 subjects enrolled in this study were included in the safety analysis set. A total of 16 subjects had 62 AEs during the study period. No serious adverse events (SAE) were reported. Most treatment-emergent adverse events were grade 1 or 2 based on CTCAE. Only one grade 3 adverse event was observed. Concomitant intake of fuzuloparib with the moderate CYP3A inhibitor efavirenz resulted in a decrease in fuzuloparib AUC0−∞ and Cmax of 78% and 53% respectively. The results suggested that concomitant moderate CYP3A inducers should be avoided during the administration of fuzuloparib, or else the dosage adjustments should be required. (This trial was registered at http://www.chinadrugtrials.org.cn. The registration No. is CTR20211022, and the date of registration is 2021-05-13).


Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Slade D (2020) PARP and PARG inhibitors in cancer treatment. Genes Dev 34(5–6):360–394. https://doi.org/10.1101/gad.334516.119
Lee A (2021) Fuzuloparib: first approval. Drugs 81(10):1221–1226. https://doi.org/10.1007/s40265-021-01541-x
Wang L, Yang C, Xie C, Jiang J, Gao M, Fu L, Li Y, Bao X, Fu H, Lou L (2019) Pharmacologic characterization of fluzoparib, a novel poly(ADP-ribose) polymerase inhibitor undergoing clinical trials. Cancer Sci 110(3):1064–1075. https://doi.org/10.1111/cas.13947
Li N, Liu Q, Tian Y, Wu L (2022) Overview of fuzuloparib in the treatment of ovarian cancer: background and future perspective. J Gynecol Oncol 33(6):e86. https://doi.org/10.3802/jgo.2022.33.e86
Wu M, Li X, Sun J, Chen H, Ding Y (2021) A phase I study of fluzoparib tablet formulation, an oral PARP inhibitor: effect of food on the pharmacokinetics and metabolism after oral dosing in healthy chinese volunteers. Expert Opin Drug Metab Toxicol 17(4):503–508. https://doi.org/10.1080/17425255.2021.1881480
Li H, Liu R, Shao B, Ran R, Song G, Wang K, Shi Y, Liu J, Hu W, Chen F et al (2020) Phase I dose-escalation and expansion study of PARP inhibitor, fluzoparib (SHR3162), in patients with advanced solid tumors. Chin J Cancer Res 32(3):370–382. https://doi.org/10.21147/j.issn.1000-9604.2020.03.08
Dirix L, Swaisland H, Verheul HM, Rottey S, Leunen K, Jerusalem G, Rolfo C, Nielsen D, Molife LR, Kristeleit R et al (2016) Effect of Itraconazole and Rifampin on the pharmacokinetics of Olaparib in patients with Advanced Solid Tumors: results of two phase I open-label studies. Clin Ther 38(10):2286–2299. https://doi.org/10.1016/j.clinthera.2016.08.010
Liao M, Jaw-Tsai S, Beltman J, Simmons AD, Harding TC, Xiao JJ (2020) Evaluation of in vitro absorption, distribution, metabolism, and excretion and assessment of drug-drug interaction of rucaparib, an orally potent poly(ADP-ribose) polymerase inhibitor. Xenobiotica 50(9):1032–1042. https://doi.org/10.1080/00498254.2020.1737759
Mu S, Lin C, Skrzypczyk-Ostaszewicz A, Bulat I, Maglakelidze M, Skarbova V, Andreu-Vieyra C, Sahasranaman S (2021) The pharmacokinetics of pamiparib in the presence of a strong CYP3A inhibitor (itraconazole) and strong CYP3A inducer (rifampin) in patients with solid tumors: an open-label, parallel-group phase 1 study. Cancer Chemother Pharmacol 88(1):81–88. https://doi.org/10.1007/s00280-021-04253-x
Chen X, Yang F, Zhao J, Tang Q, Heng J, Deng J, Zhang J, Chen Y, Li K, Wang J (2022) Effect of fluconazole on the pharmacokinetics of fuzuloparib: an open-label, crossover study in chinese healthy male volunteers. Cancer Chemother Pharmacol 89(1):141–148. https://doi.org/10.1007/s00280-021-04376-1
Zhang Q, Kai J, Zhai Y, Xu N, Shentu J, Zhang Y, Liang Y, Wang Y, Wu L (2022) The impact of rifampicin on the pharmacokinetics of fuzuloparib in healthy chinese male volunteers. Br J Clin Pharmacol 88(1):84–90. https://doi.org/10.1111/bcp.14926
Molenaar-Kuijsten L, Van Balen DEM, Beijnen JH, Steeghs N, Huitema ADR (2021) A review of CYP3A Drug-Drug Interaction Studies: practical guidelines for patients using targeted oral anticancer drugs. Front Pharmacol 12:670862. https://doi.org/10.3389/fphar.2021.670862
Pilla Reddy V, Bui K, Scarfe G, Zhou D, Learoyd M (2019) Physiologically based pharmacokinetic modeling for Olaparib Dosing Recommendations: bridging formulations, drug interactions, and patient populations. Clin Pharmacol Ther 105(1):229–241. https://doi.org/10.1002/cpt.1103
Marzolini C, Rajoli R, Battegay M, Elzi L, Back D, Siccardi M (2017) Physiologically based pharmacokinetic modeling to Predict Drug-Drug interactions with Efavirenz Involving Simultaneous Inducing and Inhibitory Effects on Cytochromes. Clin Pharmacokinet 56(4):409–420.https://doi.org/10.1007/s40262-016-0447-7
Ngaimisi E, Mugusi S, Minzi OM, Sasi P, Riedel KD, Suda A, Ueda N, Janabi M, Mugusi F, Haefeli WE et al (2010) Long-term efavirenz autoinduction and its effect on plasma exposure in HIV patients. Clin Pharmacol Ther 88(5):676–684. https://doi.org/10.1038/clpt.2010.172
Jiang X, Tao Y, Liu Y, Shi P, Li T, Sun F, Cao Y, Wang C (2022) A randomized, open-label, two-period crossover bridging study on fuzuloparib capsules of different specifications in healthy chinese volunteers. Br J Clin Pharmacol 88(3):1087–1093. https://doi.org/10.1111/bcp.15035
Maggiolo F (2007) Efavirenz. Expert Opin Pharmacother 8(8):1137–1145. https://doi.org/10.1517/14656566.8.8.1137
Acknowledgements
This work was supported by Jiangsu Hengrui Co. Ltd. The authors thank all the patients who participated in this study and their families, as well as all the investigators and site staff who made the study possible.
Funding
This project was also supported by the National Natural Science Foundation of China (No. 81503340), and the National Natural Science Foundation of Jiangsu Province (No. BK20150645), Jiangsu Research Hospital Association for Precision medication (No. JY202035), and the CHIA TAI TIAQING Pharmaceutical Foundation of Jiangsu Pharmaceutical Association (No. Q202203). We thank staff involved.
Author information
Authors and Affiliations
Contributions
Huiping Wang and Linlin Hu contributed to the study conception and design. Material preparation, data collection and analysis were performed by Linlin Hu, Ting Dou, Qiu yue Sun, Lu Tang, Ming-min Cai and Wei Qian. The first draft of the manuscript was written by Linlin Hu and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Informed consent
Informed consent was obtained from all individual participants included in the study.
Research involving human participants and/or animals
This study was performed in line with the principles of the Declaration of Helsinki. The study protocol was approved (approval number, 2021ZDSYLL079-P01) by the Ethics Committee of the Zhongda Hospital, Medical School, Southeast University (Nanjing, China).
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, L., Dou, T., Sun, Q. et al. Effect of a moderate CYP3A inducer efavirenz on the pharmacokinetics of fuzuloparib: An open-label, fixed sequence study in Chinese healthy male subjects. Invest New Drugs 41, 276–283 (2023). https://doi.org/10.1007/s10637-023-01331-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-023-01331-0