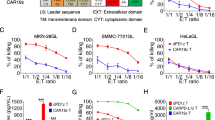
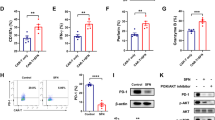
Summary
Purpose Programmed cell death 1 (PD-1), which is upregulated under the continuous induction of the tumor microenvironment, causes chimeric antigen receptor (CAR)-T cell hypofunction via interaction with programmed death ligand 1 (PD-L1). This study aimed to construct CAR-T cells that are resistant to PD-1 inhibition to improve the effect of CAR-T cells in solid tumors. Methods We constructed a type of dual-function CAR-T cell that targets tumor-associated antigen c-Met and blocks the binding of PD-1 with PD-L1. The expression of c-Met, PD-L1, and inhibitory receptors was measured using flow cytometry. The cytotoxicity, cytokine release, and differentiation level of CAR-T cells were determined using lactate dehydrogenase release assay, enzyme-linked immunosorbent assay, and flow cytometry, respectively. The levels of p-Akt, p-MAPK, caspase-3, and Bcl2 were detected by western blot. The in vivo anti-tumor effect was evaluated using tumor xenograft models. Results Dual-function CAR-T cells could mediate enhanced active signals upon encountering target antigens and had targeted cytotoxicity to target cells. However, the cytotoxicity of c-Met-CAR-PD-1+ T cells was impaired due to the interaction of PD-1 with PD-L1. By blocking the binding of PD-1 and PD-L1, the novel dual-function CAR-PD-1+ T cells could maintain cytotoxicity to PD-L1+ tumor cells. In tumor tissue, the dual-function CAR-T cells showed lower inhibitory receptor expression and lower differentiation characteristics, which resulted in potent anti-tumor effects and prolonged survival in PD-L1+ tumor xenograft models compared to single-target CAR-T cells. Conclusion These results confirm that the novel dual-function CAR-T cells exhibit stronger anti-tumor activity against solid tumors than traditional single-target CAR-T cells and present a new approach that enhance the activity of CAR-T cells in solid tumors.







Similar content being viewed by others
References
Lim WA, June CH (2017) The principles of engineering immune cells to treat cancer. Cell 168(4):724–740. https://doi.org/10.1016/j.cell.2017.01.016
Kershaw MH, Westwood JA, Darcy PK (2013) Gene-engineered T cells for cancer therapy. Nat Rev Cancer 13(8):525–541. https://doi.org/10.1038/nrc3565
Park JH, Geyer MB, Brentjens RJ (2016) CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood 127(26):3312–3320. https://doi.org/10.1182/blood-2016-02-629063
Garfall AL, Maus MV, Hwang WT, Lacey SF, Mahnke YD, Melenhorst JJ, Zheng Z, Vogl DT, Cohen AD, Weiss BM, Dengel K, Kerr ND, Bagg A, Levine BL, June CH, Stadtmauer EA (2015) Chimeric antigen receptor T cells against CD19 for multiple myeloma. N Engl J Med 373(11):1040–1047. https://doi.org/10.1056/NEJMoa1504542
Yong CSM, Dardalhon V, Devaud C, Taylor N, Darcy PK, Kershaw MH (2017) CAR T cell therapy of solid tumors. Immunol Cell Biol 95(4):356–363. https://doi.org/10.1038/icb.2016.128
Zhang C, Wang Z, Yang Z, Wang M, Li S, Li Y, Zhang R, Xiong Z, Wei Z, Shen J, Luo Y, Zhang Q, Liu L, Qin H, Liu W, Wu F, Chen W, Pan F, Zhang X, Bie P, Liang H, Pecher G, Qian C (2017) Phase I escalating-dose trial of CAR-T therapy targeting CEA + metastatic colorectal cancers. Mol Ther 25(5):1248–1258. https://doi.org/10.1016/j.ymthe.2017.03.010
Yee C (2018) Adoptive T cell therapy: points to consider. Curr Opin Immunol 51:197–203. https://doi.org/10.1016/j.coi.2018.04.007
Xia AL, Wang XC, Lu YJ, Lu XJ, Sun B (2017) Chimeric-antigen receptor T (CAR-T) cell therapy for solid tumors: challenges and opportunities. Oncotarget 8(52):90521–90531. https://doi.org/10.18632/oncotarget.19361
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L (2002) Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8(8):793–800. https://doi.org/10.1038/nm730
Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L (2007) Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 4(127):127ra37. https://doi.org/10.1126/scitranslmed.3003689
Bardhan K, Anagnostou T, Boussiotis VA (2016) The PD1: PD-L1/2 pathway from discovery to clinical implementation. Front Immunol 7:550. https://doi.org/10.3389/fimmu.2016.00550
Motz GT, Coukos G (2013) Deciphering and reversing tumor immune suppression. Immunity 39(1):61–73. https://doi.org/10.1016/j.immuni.2013.07.005
Jiang Y, Li Y, Zhu B (2015) T-cell exhaustion in the tumor microenvironment. Cell Death Dis 6(6):e1792. https://doi.org/10.1038/cddis.2015.162
Scarfo I, Maus MV (2017) Current approaches to increase CAR T cell potency in solid tumors: targeting the tumor microenvironment. J Immunother Cancer 5:28. https://doi.org/10.1186/s40425-017-0230-9
du Rusquec P, de Calbiac O, Robert M, Campone M, Frenel JS (2019) Clinical utility of pembrolizumab in the management of advanced solid tumors: an evidence-based review on the emerging new data. Cancer Manag Res 11:4297–4312. https://doi.org/10.2147/CMAR.S151023
Zou W, Wolchok JD, Chen L (2016) PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med 8(328):328rv4. https://doi.org/10.1126/scitranslmed.aad7118
Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, Eder JP, Balmanoukian AS, Aggarwal C, Horn L, Patnaik A, Gubens M, Ramalingam SS, Felip E, Goldman JW, Scalzo C, Jensen E, Kush DA, Hui R (2019) Five-year overall survival for patients with advanced non–small-cell lung Cancer treated with Pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol 37(28):2518–2527. https://doi.org/10.1200/JCO.19.00934
Shen X, Zhao B (2018) Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ 362:k3529. https://doi.org/10.1136/bmj.k3529
Postow MA, Sidlow R, Hellmann MD (2016) Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378(2):158–168. https://doi.org/10.1056/NEJMra1703481
Liu X, Ranganathan R, Jiang S, Fang C, Sun J, Kim S, Newick K, Lo A, June CH, Zhao Y, Moon EK (2016) A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res 76(6):1578–1590. https://doi.org/10.1158/0008-5472.CAN-15-2524
Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, Sadelain M, Adusumilli PS (2016) Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest 126(8):3130–3144. https://doi.org/10.1172/JCI83092
Bouattour M, Raymond E, Qin S, Cheng AL, Stammberger U, Locatelli G, Faivre S (2018) Recent developments of c-Met as a therapeutic target in hepatocellular carcinoma. Hepatology 67(3):1132–1149. https://doi.org/10.1002/hep.29496
Pasquini G, Giaccone G (2018) C-MET inhibitors for advanced non-small cell lung cancer. Expert Opin Investig Drugs 27(4):363–375. https://doi.org/10.1080/13543784.2018.1462336
Bradley CA, Salto-Tellez M, Laurent-Puig P, Bardelli A, Rolfo C, Tabernero J, Khawaja HA, Lawler M, Johnston PG, Van Schaeybroeck S (2017) Targeting c-MET in gastrointestinal tumours: rationale, opportunities and challenges. Nat Rev Clin Oncol 14(9):562–576. https://doi.org/10.1038/nrclinonc.2017.40
Wang W, Dong J, Wang M, Yao S, Tian X, Cui X, Fu S, Zhang S (2018) miR-148a-3p suppresses epithelial ovarian cancer progression primarily by targeting c-Met. Oncol Lett 15(5):6131–6136. https://doi.org/10.3892/ol.2018.8110
Anestis A, Zoi I, Karamouzis MV (2018) Current advances of targeting HGF/c-Met pathway in gastric cancer. Ann Transl Med 6(12):247. https://doi.org/10.21037/atm.2018.04.42
Park CH, Cho SY, Ha JD, Jung H, Kim HR, Lee CO, Jang IY, Chae CH, Lee HK, Choi SU (2016) Novel c-Met inhibitor suppresses the growth of c-Met-addicted gastric cancer cells. BMC Cancer 16:35. https://doi.org/10.1186/s12885-016-2058-y
Hsieh YS, Liao CH, Chen WS, Pai JT, Weng MS (2017) Shikonin inhibited migration and invasion of human lung Cancer cells via suppression of c-Met-mediated epithelial-to-Mesenchymal transition. J Cell Biochem 118(12):4639–4651. https://doi.org/10.1002/jcb.26128
Lee D, Sung ES, Ahn JH, An S, Huh J, You WK (2015) Development of antibody-based c-Met inhibitors for targeted cancer therapy. Immunotargets Ther 4:35–44. https://doi.org/10.2147/ITT.S37409
Li S, Siriwon N, Zhang X, Yang S, Jin T, He F, Kim YJ, Mac J, Lu Z, Wang S, Han X, Wang P (2017) Enhanced cancer immunotherapy by chimeric antigen receptor-modified T cells engineered to secrete checkpoint inhibitors. Clin Cancer Res 23(22):6982–6992. https://doi.org/10.1158/1078-0432.CCR-17-0867
Papaioannou NE, Beniata OV, Vitsos P, Tsitsilonis O, Samara P (2016) Harnessing the immune system to improve cancer therapy. Ann Transl Med 4(14):261. https://doi.org/10.21037/atm.2016.04.01
Gargett T, Yu W, Dotti G, Yvon ES, Christo SN, Hayball JD, Lewis ID, Brenner MK, Brown MP (2016) GD2-specific CAR T cells undergo potent activation and deletion following antigen encounter but can be protected from activation-induced cell death by PD-1 blockade. Mol Ther 24(6):1135–1149. https://doi.org/10.1038/mt.2016.63
Alizadeh D, Wong RA, Yang X, Wang D, Pecoraro JR, Kuo C-F, Aguilar B, Qi Y, Ann DK, Starr R, Urak R, Wang X, Forman SJ, Brown CE (2019) IL15 enhances CAR-T cell antitumor activity by reducing mTORC1 activity and preserving their stem cell memory phenotype. Cancer Immunol Res 7(5):759–772. https://doi.org/10.1158/2326-6066.CIR-18-0466
Hadrup S, Donia M, Thor Straten P (2013) Effector CD4 and CD8 T cells and their role in the tumor microenvironment. Cancer Microenviron 6(2):123–133. https://doi.org/10.1007/s12307-012-0127-6
Xie YJ, Dougan M, Jailkhani N, Ingram J, Fang T, Kummer L, Momin N, Pishesha N, Rickelt S, Hynes RO, Ploegh H (2019) Nanobody-based CAR T cells that target the tumor microenvironment inhibit the growth of solid tumors in immunocompetent mice. PNAS 116(16):7624–7631. https://doi.org/10.1073/pnas.1817147116
Mardiana S, Solomon BJ, Darcy PK, Beavis PA (2019) Supercharging adoptive T cell therapy to overcome solid tumor-induced immunosuppression. Sci Transl Med 11(495):eaaw2293. https://doi.org/10.1126/scitranslmed.aaw2293
Yin Y, Boesteanu AC, Binder ZA, Xu C, Reid RA, Rodriguez JL, Cook DR, Thokala R, Blouch K, McGettigan-Croce B, Zhang L, Konradt C, Cogdill AP, Panjwani MK, Jiang S, Migliorini D, Dahmane N, Posey AD Jr, June CH, Mason NJ, Lin Z, O'Rourke DM, Johnson LA (2018) Checkpoint blockade reverses Anergy in IL-13Rα2 humanized scFv-based CAR T cells to treat murine and canine Gliomas. Mol Ther Oncolytics 11:20–38. https://doi.org/10.1016/j.omto.2018.08.002
Kosti P, Maher J, Arnold JN (2018) Perspectives on chimeric antigen receptor T-cell immunotherapy for solid tumors. Front Immunol 9:1104. https://doi.org/10.3389/fimmu.2018.01104
Moon EK, Wang LC, Dolfi DV, Wilson CB, Ranganathan R, Sun J, Kapoor V, Scholler J, Puré E, Milone MC, June CH, Riley JL, Wherry EJ, Albelda SM (2014) Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin Cancer Res 20:4262–4273. https://doi.org/10.1158/1078-0432.CCR-13-2627
Gao Y, Shi S, Ma W, Chen J, Cai Y, Ge L, Li L, Wu J, Tian J (2019) Bibliometric analysis of global research on PD-1 and PD-L1 in the field of cancer. Int Immunopharmacol 72:374–384. https://doi.org/10.1016/j.intimp.2019.03.045
John LB, Devaud C, Duong CP, Yong CS, Beavis PA, Haynes NM, Chow MT, Smyth MJ, Kershaw MH, Darcy PK (2018) Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res 19:5636–5646. https://doi.org/10.1158/1078-0432.CCR-13-0458
Yoon DH, Osborn MJ, Tolar J, Kim CJ (2018) Incorporation of immune checkpoint blockade into chimeric antigen receptor T cells (CAR-Ts): combination or built-in CAR-T. Int J Mol Sci 19(2):E340. https://doi.org/10.3390/ijms19020340
Heczey A, Louis CU, Savoldo B, Dakhova O, Durett A, Grilley B, Liu H, Wu MF, Mei Z, Gee A, Mehta B, Zhang H, Mahmood N, Tashiro H, Heslop HE, Dotti G, Rooney CM, Brenner MK (2017) CAR T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol Therapy 25(9):2214–2224. https://doi.org/10.1016/j.ymthe.2017.05.012
Smith TT, Moffett HF, Stephan SB, Opel CF, Dumigan AG, Jiang X, Pillarisetty VG, Pillai SPS, Wittrup KD, Stephan MT (2017) Biopolymers codelivering engineered T cells and STING agonists can eliminate heterogeneous tumors. J Clin Invest 127(6):2176–2191. https://doi.org/10.1172/JCI87624
Haji-Fatahaliha M, Hosseini M, Akbarian A, Sadreddini S, Jadidi-Niaragh F, Yousefi M (2016) CAR-modified T-cell therapy for cancer: an updated review. Artif Cells Nanomed Biotechnol 44(6):1339–1349. https://doi.org/10.3109/21691401.2015.1052465
Rupp LJ, Schumann K, Roybal KT, Gate RE, Ye CJ, Lim WA, Marson A (2017) CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci Rep 7(1):737. https://doi.org/10.1038/s41598-017-00462-8
Hu W, Zi Z, Jin Y, Li G, Shao K, Cai Q, Ma X, Wei F (2019) CRISPR/Cas9-mediated PD-1 disruption enhances human mesothelin-targeted CAR T cell effector functions. Cancer Immunol Immunother 68(3):365–377. https://doi.org/10.1007/s00262-018-2281-2
Li S, Siriwon N, Zhang X, Yang S, Jin T, He F, Kim YJ, Mac J, Lu Z, Wang S, Han X, Wang P (2017) Enhanced Cancer immunotherapy by chimeric antigen receptor-modified T cells engineered to secrete checkpoint inhibitors. Clin Cancer Res 23(22):6982–6992. https://doi.org/10.1158/1078-0432.CCR-17-0867
Rafiq S, Yeku OO, Jackson HJ, Purdon TJ, van Leeuwen DG, Drakes DJ, Song M, Miele MM, Li Z, Wang P, Yan S, Xiang J, Ma X, Seshan VE, Hendrickson RC, Liu C, Brentjens RJ (2018) Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol 36(9):847–856. https://doi.org/10.1038/nbt.4195
Los M, Van de Craen M, Penning LC et al (1995) Requirement of an ICE/CED-3 protease for Fas/APO-1-mediated apoptosis. Nature 375(6526):81–83. https://doi.org/10.1038/375081a0
Acknowledgments
We thank Yao Wang from Key Laboratory of Medical Molecular Virology, Ministry of Education and Public Health, School of Basic Medical Sciences, Fudan University for help in flow cytometry.
Funding
This study was funded by the National Key Research Project Bio-safety Key Technology Development Program 2016YFC1201501 and the National Natural Science Foundation of China, No. 31671228.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Min Yu, Wei Mo, Huijie Wang, Zujun Sun and Xingxing Yuan designed the study. Xingxing Yuan performed all the material preparation, experiments and wrote the manuscript. Qingyun Yuan, Qiaoyan Liang, Weihua Hou and Yuxiong Wang assisted with all in vitro experiments. Qingyun Yuan contributed to the in vivo experiments. Xingxing Yuan performed data collection, analysis and interpretation. The first draft of the manuscript was written by Xingxing Yuan and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All procedures performed in studies involving animals were in accordance with the ethical standards of the department of laboratory animal science of Fudan university and permitted on March 6, 2019.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
Supplementary Fig. 1 The detection of c-Met and PD-L1 in tumor cell lines. (a) The expression of c-Met in each tumor cell line detected by western blot. (b) Flow cytometric analysis of the expression of c-Met in four tumor cell lines. (c) Flow cytometry histogram of PD-L1 expression in four tumor cell lines and PD-L1 up-regulation after stimulation with IFN-γ (40 μg/mL) for 8 h. Supplementary Fig. 2 Representative flow cytometry contour plots of the proportion of CAR-T cells expressing PD-1, LAG-3, and TIM-3 in each group on days 8, 16, and 24 during long-term stimulation. Supplementary Fig. 3 Analysis of tumor-infiltrating CAR-T cells. (a) Quantification of the proportions of perforin+ and granzyme B+ cells within the tumor-infiltrating CAR-T cells of each group 30 days after treatment with CAR-T cells analyzed by flow cytometry (n = 3, mean ± SD, ****P < 0.0001 by one-way ANOVA). (b) Quantification of the inhibitory receptors (PD-1, LAG-3, and TIM-3) on the tumor-infiltrating CAR-T cells 30 days after treatment with CAR-T cells (n = 3, mean ± SD, n.s.: not significant, ***P < 0.001 by one-way ANOVA). (c) Pie charts summarizing the proportion of CD45RA+CD62L+ cells in the tumor-infiltrating CAR-T cells 15 and 30 days after treatment with CAR-T cells (top). Quantification of the proportion of CD62L+CD45RA+ cells in the tumor-infiltrating CAR-T cells (bottom) (n = 3, mean ± SD, ****P < 0.0001 by two-way ANOVA). (d) The frequency of CD8+ cells in tumor-infiltrating CAR-T cells extracted from each group 15 and 30 days after treatment (top). Quantification of the percentages of CD8+ T cells (bottom) (n = 3, mean ± SD, ***P < 0.001, ****P < 0.0001 by two-way ANOVA). (e) The cytotoxicity (left) and IFN-γ secretion (right) of the tumor-infiltrating CAR-T cells in each group were detected in comparison with the original c-Met-PD-1-CAR-T cells (n = 3, mean ± SD, ****P < 0.0001 by two-way ANOVA) (PDF 33429 kb)
Rights and permissions
About this article
Cite this article
Yuan, X., Sun, Z., Yuan, Q. et al. Dual-function chimeric antigen receptor T cells targeting c-Met and PD-1 exhibit potent anti-tumor efficacy in solid tumors. Invest New Drugs 39, 34–51 (2021). https://doi.org/10.1007/s10637-020-00978-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-020-00978-3