Abstract
Background and Aims
Variation in colorectal neoplasia detection limits the effectiveness of screening colonoscopy. By evaluating neoplasia detection rates of individual colonoscopists, we aimed to quantify the effects of pre-procedural knowledge of a positive (+) multi-target stool DNA (mt-sDNA) on colonoscopy quality metrics.
Methods
We retrospectively identified physicians who performed a high volume of + mt-sDNA colonoscopies; colorectal neoplasia at post-mt-sDNA colonoscopy was recorded. These colonoscopists were stratified into quartiles based on baseline adenoma detection rates. Baseline colonoscopy adenoma detection rates and sessile serrated lesion detection rates were compared to post-mt-sDNA colonoscopy neoplasia diagnosis rates among each quartile. Withdrawal times were measured from negative exams.
Results
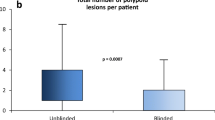
During the study period (2014–17) the highest quartile of physicians by volume of post-mt-sDNA colonoscopies were evaluated. Among thirty-five gastroenterologists, their median screening colonoscopy adenoma detection rate was 32% (IQR, 28–39%) and serrated lesion detection rate was 13% (8–15%). After + mt-sDNA, adenoma diagnosis increased to 47% (36–56%) and serrated lesion diagnosis increased to 31% (17–42%) (both p < 0.0001). Median withdrawal time increased from 10 (7–13) to 12 (10–17) minutes (p < 0.0001) and was proportionate across quartiles. After + mt-sDNA, lower baseline detectors had disproportionately higher rates of adenoma diagnosis in female versus male patients (p = 0.048) and higher serrated neoplasia diagnosis rates among all patients (p = 0.0092).
Conclusions
Knowledge of + mt-sDNA enriches neoplasia diagnosis compared to average risk screening exams. Adenomatous and serrated lesion diagnosis was magnified among those with lower adenoma detection rates. Awareness of the mt-sDNA result may increase physician attention during colonoscopy.
Graphical Abstract
Pre-procedure knowledge of a positive mt-sDNA test improves neoplasia diagnosis rates among colonoscopists with lower baseline adenoma detection rates, independent of withdrawal time.






Similar content being viewed by others
Abbreviations
- CRN:
-
Colorectal neoplasia
- mt-sDNA:
-
Multi-target stool DNA
- ADR:
-
Adenoma detection rate
- SDR:
-
Serrated lesion detection rate
- CRC:
-
Colorectal cancer
- SSL:
-
Sessile serrated lesion
- IQR:
-
Interquartile range
- CI:
-
Confidence intervals
- FIT:
-
Fecal immunochemical test
References
Siegel RL, Miller KD, Fuchs HE et al. Cancer statistics, 2022. CA: Cancer Journal for Clinicians 2022;72:7–33.
Davidson KW, Barry MJ, Mangione CM et al. Screening for colorectal cancer: US preventive services task force recommendation statement. Jama 2021;325:1965–1977.
Klabunde CN, Djenaba JA, King JB, White A, Plescia M. Vital signs: colorectal cancer screening test use-United States, 2012. CDC MMWR-Morbid Mortal W 2013;62:881–888.
Winawer SJ, Zauber AG, Ho MN et al. Prevention of colorectal cancer by colonoscopic polypectomy. The national polyp study workgroup. The New England Journal of Medicine 1993;329:1977–1981.
Barclay RL, Vicari JJ, Doughty AS et al. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med 2006;355:2533–2541.
Chen SC, Rex DK. Endoscopist can be more powerful than age and male gender in predicting adenoma detection at colonoscopy. Am J Gastroenterol 2007;102:856–861.
Barclay RL, Vicari JJ, Doughty AS et al. Serrated and adenomatous polyp detection increases with longer withdrawal time: results from the New Hampshire Colonoscopy Registry. Am J Gastroenterol 2014;109:417–426.
Corley DA, Jensen CD, Marks AR et al. Adenoma detection rate and risk of colorectal cancer and death. New England Journal of Medicine 2014;370:1298–1306.
Kaminski MF, Regula J, Kraszewska E et al. Quality indicators for colonoscopy and the risk of interval cancer. New England Journal of Medicine 2010;362:1795–1803.
Stegmaier C, Brenner H, Altenhofen L et al. Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study. JNCI: Journal of the National Cancer Institute. 2010;102:89–95.
Baxter NN, Warren JL, Barrett MJ et al. Association between colonoscopy and colorectal cancer mortality in a US cohort according to site of cancer and colonoscopist specialty. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 2012;30:2664–9.
Nishihara R, Wu K, Lochhead P et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. The New England journal of medicine. 2013;369:1095–1105.
Kim SY, Lee SJ, Chung JW et al. Efficacy of repeat forward-view examination of the right-sided colon during colonoscopy: A prospective randomized controlled trial. J Gastroenterol Hepatol. 2020. https://doi.org/10.1111/jgh.15064.
Cubiella J, Castells A, Andreu M et al. Correlation between adenoma detection rate in colonoscopy- and fecal immunochemical testing-based colorectal cancer screening programs. United European Gastroenterology Journal 2017;5:255–60.
Robertson DJ, Lee JK, Boland CR et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US multi-society task force on colorectal cancer. Gastroenterology 2017;152:1217–37.e3.
Kligman E, Li W, Eckert GJ et al. Adenoma detection rate in asymptomatic patients with positive fecal immunochemical tests. Digestive Diseases and Sciences 2018;63:1167–72.
Anderson JC, Robinson CM, Hisey W et al. Colonoscopy findings in FIT+ and mt-sDNA+ patients versus in colonoscopy-only patients: new hampshire colonoscopy registry data. Cancer Prev Res (Phila). 2022;15:455–64.
Imperiale TF, Ransohoff DF, Itzkowitz SH et al. Multitarget stool DNA testing for colorectal-cancer screening. New England Journal of Medicine 2014;370:1287–97.
Johnson DH, Kisiel JB, Burger KN et al. Multitarget stool DNA test: clinical performance and impact on yield and quality of colonoscopy for colorectal cancer screening. Gastrointest Endosc 2017;85:657-665.e1.
Eckmann JD, Ebner DW, Bering J et al. Multitarget stool DNA screening in clinical practice: high positive predictive value for colorectal neoplasia regardless of exposure to previous colonoscopy. Am J Gastroenterol 2020;115:608–615.
Gupta S, Lieberman D, Anderson JC et al. Recommendations for follow-up after colonoscopy and polypectomy: a consensus update by the us multi-society task force on colorectal cancer. Official Journal of the American College of Gastroenterology/ACG 2020;115:415–34.
Ezaz G, Leffler DA, Beach S et al. Association between endoscopist personality and rate of adenoma detection. Clin Gastroenterol Hepatol 2019;17:1571-1579.e7.
Dik VK et al. Measuring gaze patterns during colonoscopy: a useful tool to evaluate colon inspection? European Journal of Gastroenterology & Hepatology 2016;28:1400–1406.
Mehrotra A, Morris M, Gourevitch RA et al. Physician characteristics associated with higher adenoma detection rate. Gastrointest Endosc 2018;87:778-786.e5.
Rex DK, Ahnen DJ, Baron JA et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Official Journal of the American College of Gastroenterology/ACG 2012;107:1315–29.
Wong JCT, Chiu HM, Kim HS et al. Adenoma detection rates in colonoscopies for positive fecal immunochemical tests versus direct screening colonoscopies. Gastrointestinal Endoscopy 2019;89:607-613.e1.
Chang L-C, Shun C-T, Hsu W-F et al. Fecal immunochemical test detects sessile serrated adenomas and polyps with a low level of sensitivity. Clinical Gastroenterology and Hepatology 2017;15:872-879.e1.
Bosch LJW, Melotte V, Mongera S et al. Multitarget stool DNA test performance in an average-risk colorectal cancer screening population. Am J Gastroenterol 2019;114:1909–1918.
van Toledo D, Jeg IJ, Bossuyt PMM et al. Serrated polyp detection and risk of interval post-colonoscopy colorectal cancer: a population-based study. Lancet Gastroenterol Hepatol 2022;7:747–54.
Anderson JC, Hisey WM, Robinson CM et al. Serrated polyp yield at colonoscopy in patients with positive FIT, positive mt-sDNA, and colonoscopy only: data from the new hampshire colonoscopy registry. Cancer Epidemiol Biomarkers Prev 2023;32:226–232.
Kahi CJ, Li X, Eckert GJ et al. High colonoscopic prevalence of proximal colon serrated polyps in average-risk men and women. Gastrointestinal Endoscopy 2012;75:515–520.
Rex DK, Schoenfeld PS, Cohen J et al. Quality indicators for colonoscopy. Gastrointestinal Endoscopy 2015;81:31–53.
A Ahlquist D. Aberrantly methylated gene marker levels in stool: effects of demographic, exposure, body mass, and other patient characteristics. Journal of Molecular Biomarkers & Diagnosis. 2012. https://doi.org/10.4172/2155-9929.1000133.
Funding
This work was supported by a grant from the National Institutes of Health (CA214679, to Kisiel).
Author information
Authors and Affiliations
Contributions
DWE: Data curation, investigation, writing of original draft, lead. KNB: Data curation, formal analysis, project administration, writing- review and editing, supporting. DWM: Data curation, formal analysis, methodology, validation, writing-review and editing, supporting. BTB: Data curation, formal analysis, writing-review and editing, supporting. JDE: Data curation, writing-review and editing, supporting. MED: Data curation, writing-review and editing, supporting. KLL: Data curation, writing-review and editing, supporting. JBL: Data curation, methodology, writing-review and editing, supporting. JB: Data curation, writing-review and editing, supporting. AK: Data curation, writing-review and editing, supporting. EAR: Data curation, writing-review and editing, supporting. DOP: Methodology, writing-review and editing, supporting. MBW: Methodology, writing-review and editing, supporting. SVK: Methodology, writing-review and editing, supporting. JAL: Methodology, writing-review and editing, supporting. NSB: Methodology, writing-review and editing, supporting. LJFR: Methodology, writing-review and editing, supporting. SRG: Methodology, writing-review and editing, supporting. JBK: Conceptualization, funding acquisition, methodology, lead, writing-review and editing, supporting.
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Ebner has a professional service agreement with Exact Sciences (Madison, WI) serving as an independent contractor to provide guidance on study design and analysis. Dr. Kisiel and Mr. Mahoney are listed as inventors of Mayo Clinic intellectual property licensed to Exact Sciences and have rights to receive royalties, paid to Mayo Clinic. Dr. Finney Rutten was previously an Employee of Exact Sciences. No other authors have potential conflicts to disclose.
Ethical approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of Mayo Clinic approved this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ebner, D.W., Burger, K.N., Mahoney, D.W. et al. Neoplasia Diagnosis After Multi-target Stool DNA Is Enhanced Among Lowest Baseline Detectors. Dig Dis Sci 68, 3721–3731 (2023). https://doi.org/10.1007/s10620-023-08038-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-08038-5