Abstract
Background
Non-invasive tools including liver stiffness measurement (LSM) or FIB-4, assessed before or after direct acting antivirals (DAA), have been suggested to predict hepatocellular carcinoma (HCC).
Aims
This study aims to compare predictability of HCC by these methods at different time points, to validate the HCC surveillance suggestion by guidelines, and to propose personalized strategy.
Methods
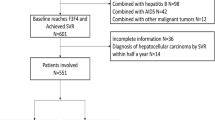
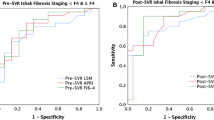
Chronic hepatitis C whose LSM and FIB-4 were available at pretherapy and after sustained virological response (SVR) were enrolled. Advanced chronic liver disease (ACLD) was defined as pretherapy LSM ≥ 10 kPa or FIB-4 index ≥ 3.25 or ultrasound signs of cirrhosis plus platelet count < 150,000/μL. The predictabilities were compared by area under ROC. The cumulative HCC incidences were calculated by Kaplan–Meier analysis.
Results
Among 466 ACLD patients, 40 patients developed HCC during a follow-up duration of 26.8 months. Comparable predictive performances for HCC between LSM and FIB-4 at pretherapy and SVR were noted. By guidelines suggestion using pretherapy LSM = 10 kPa (advanced fibrosis) and 13 kPa (cirrhosis) for risk stratification, the annual HCC incidences of those with LSM of < 10, 10–12.9 and ≥ 13 kPa were 1.1, 3.6, and 5.0%, respectively. Combination of baseline LSM < 12 kPa and SVR FIB-4 < 3.7 could further stratify relatively low risk of HCC in ACLD patients of annal incidence of 1.2%.
Conclusions
ACLD patients who met advanced fibrosis but not cirrhosis by guidelines’ cut-offs still posed high risk of HCC. Baseline LSM with SVR FIB-4 can be applied to stratify low, intermediate, and high risk of HCC for personalizing surveillance strategies after SVR.
Graphical Abstract




Similar content being viewed by others
Data availability
Data are available on request due to privacy or other restrictions by the IRB board committee of Chang Gung Memorial Hospital.
Change history
17 October 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10620-022-07683-6
Abbreviations
- ACLD:
-
Advanced chronic liver disease
- AFP:
-
Alpha-fetoprotein
- ALT:
-
Alanine aminotransferase
- AUROC:
-
Area under receiver-operating characteristic
- BMI:
-
Body mass index
- CHC:
-
Chronic hepatitis C
- CI:
-
Confidence interval
- CT:
-
Computerized tomography
- DAA:
-
Direct antiviral agents
- EOT:
-
End of treatment
- HR:
-
Hazard ratio
- HbA1c:
-
Glycohemoglobin
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- HD:
-
Hepatic decompensation
- HIV:
-
Human immunodeficiency virus
- IFN:
-
Interferon
- INR:
-
International normalized ratio
- IQR:
-
Interquartile range
- kPa:
-
Kilopascal
- LSM:
-
Liver stiffness measurement
- MELD:
-
Model for end-stage liver disease
- MRI:
-
Magnetic resonance imaging
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- SVR:
-
Sustained virological response
- TE:
-
Transient elastography
References
European Association for The Study of The Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461–511.
Ghany MG, Morgan TR. AASLD-IDSA hepatitis C guidance panel. Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology 2020;71:686–721.
Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2018;68:25–32.
Thabut D, Bureau C, Layese R et al. Validation of Baveno VI criteria for screening and surveillance of esophageal varices in patients with compensated cirrhosis and a sustained response to antiviral therapy. Gastroenterology 2019;156:e1005.
McDonald SA, Pollock KG, Barclay ST et al. Real-world impact following initiation of interferon-free hepatitis C regimens on liver-related outcomes and all-cause mortality among patients with compensated cirrhosis. J Viral Hepatitis 2020;27:270–280.
Moon AM, Green PK, Rockey DC, Berry K, Ioannou GN. Hepatitis C eradication with direct-acting anti-virals reduces the risk of variceal bleeding. Aliment Pharmacol Ther. 2020;51:364–373.
Ioannou GN. HCC surveillance after SVR in patients with F3/F4 fibrosis. J Hepatol. 2020;74:458–465.
Pons M, Rodríguez-Tajes S, Esteban JI et al. Non-invasive prediction of liver-related events in patients with HCV-associated compensated advanced chronic liver disease after oral antivirals. J Hepatol. 2020;72:472–480.
Alonso López S, Manzano ML, Gea F et al. A model based on noninvasive markers predicts very low hepatocellular carcinoma risk after viral response in hepatitis C virus-advanced fibrosis. Hepatology 2020;72:1924–1934.
Semmler G, Binter T, Kozbial K et al. Non-invasive risk stratification after HCV-eradication in patients with advanced chronic liver disease. Hepatology 2020;73:1275–1289.
Butt AA, Ren Y, Lo Re H III, Taddei TH, Kaplan DE. Comparing child-Pugh, MELD, and FIB-4 to predict clinical outcomes in hepatitis C virus-infected persons: results from ERCHIVES. Clin Infect Dis. 2017;65:64–72.
Hézode C, Castéra L, Roudot-Thoraval F et al. Liver stiffness diminishes with antiviral response in chronic hepatitis C. Aliment Pharmacol Ther. 2011;34:656–663.
Anrs C. Regression of liver stiffness after sustained hepatitis C virus (HCV) virological responses among HIV/HCV-coinfected patients. AIDS (London, England) 2015;29:1821.
De Franchis R. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–752.
Narita Y, Genda T, Tsuzura H et al. Prediction of liver stiffness hepatocellular carcinoma in chronic hepatitis C patients on interferon-based anti-viral therapy. J Gastroenterol Hepatol. 2014;29:137–143.
Wang JH, Yen YH, Yao CC et al. Liver stiffness-based score in hepatoma risk assessment for chronic hepatitis C patients after successful antiviral therapy. Liver Int. 2016;36:1793–1799.
Lee HW, Chon YE, Kim SU et al. Predicting liver-related events using transient elastography in chronic hepatitis C patients with sustained virological response. Gut Liver 2016;10:429.
Degasperi E, D’Ambrosio R, Iavarone M et al. Factors associated with increased risk of de novo or recurrent hepatocellular carcinoma in patients with cirrhosis treated with direct-acting antivirals for HCV infection. Clin Gastroenterol Hepatol 2019;17:e1187.
Rinaldi L, Guarino M, Perrella A et al. Role of liver stiffness measurement in predicting HCC occurrence in direct-acting antivirals setting: a real-life experience. Dig Dis Sci. 2019;64:3013–3019.
Peleg N, Issachar A, Sneh Arbib O et al. Liver steatosis is a major predictor of poor outcomes in chronic hepatitis C patients with sustained virological response. J Viral Hepatitis 2019;26:1257–1265.
Søholm J, Hansen JF, Mössner B et al. Low incidence of HCC in chronic hepatitis C patients with pretreatment liver stiffness measurements below 17.5 kilopascal who achieve SVR following DAAs. PLoS ONE 2020;15:0243725.
Lim JK, Flamm SL, Singh S et al. American Gastroenterological Association Institute guideline on the role of elastography in the evaluation of liver fibrosis. Gastroenterology 2017;152:1536–1543.
Degos F, Perez P, Roche B et al. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol. 2010;53:1013–1021.
Afdhal NH, Bacon BR, Patel K et al. Accuracy of fibroscan, compared with histology, in analysis of liver fibrosis in patients with hepatitis B or C: a United States multicenter study. Clin Gastroenterol Hepatol. 2015;13:e773.
Ravaioli F, Conti F, Brillanti S et al. Hepatocellular carcinoma risk assessment by the measurement of liver stiffness variations in HCV cirrhotics treated with direct acting antivirals. Digest Liver Dis. 2018;50:573–579.
Broquetas T, Herruzo-Pino P, Mariño Z et al. Elastography is unable to exclude cirrhosis after sustained virological response in HCV-infected patients with advanced chronic liver disease. Liver Int. 2021;41:2733–2746.
Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology 2017;153:e1001.
Ioannou GN, Beste LA, Green PK et al. Increased risk for hepatocellular carcinoma persists up to 10 years after HCV eradication in patients with baseline cirrhosis or high FIB-4 scores. Gastroenterology 2019;157:e1264.
Facciorusso A, Del Prete V, Turco A, Buccino RV, Nacchiero MC, Muscatiello N. Long-term liver stiffness assessment in hepatitis C virus patients undergoing antiviral therapy: Results from a 5-year cohort study. J Gastroenterol Hepatol. 2018;33:942–949.
Singh S, Facciorusso A, Loomba R, Falck-Ytter YT. Magnitude and kinetics of decrease in liver stiffness after antiviral therapy in patients with chronic hepatitis C: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16:e24.
Acknowledgments
We have to thank Ms. Hui-Chuan Cheng, Ms. Yi-Meng Chiang, and Ms. Pei-Chueh Li for their assistance in database creation and maintenance.
Funding
This study was supported by grants from Chang Gung Medical Research Fund (CMRPG3I0271-2, CMRPG3K0891 and CMRPG3M1131).
Author information
Authors and Affiliations
Contributions
Conceptualization: W-JJ, C-YL; Methodology: W-JJ, Y-CL, Y-TC, Y-CH, Y-CC, R-NC, D-IT, C-YL; Data recruitment: Y-CL, Y-TC, Y-CH, D-IT, I-SS; formal analysis and investigation: Y-CL, W-JJ; Writing—original draft preparation: Y-CL; Writing—review and editing: W-JJ and all the authors; Funding acquisition: W-JJ; Resources: C-YL, D-IT, I-SS; Supervision: W-JJeng.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or personal relationships with other people or organizations that could inappropriately influence (bias) their work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An editorial commenting on this article is available at https://doi.org/10.1007/s10620-022-07623-4.
The original online version of this article was revised: The following text was inadvertently included in the article title: ‘Characters: 102(<120)’. However, now the article title is corrected.
Supplementary Information
Below is the link to the electronic supplementary material.
10620_2022_7621_MOESM2_ESM.tif
Supplementary figure 2 Comparisons of predictive performance for HCC with AUROC curves between LSM/FIB-4 at different time points (a) baseline LSM and baseline FIB-4 (b) SVR LSM and SVR FIB-4 (c) baseline LSM and SVR LSM (TIF 138 kb)
10620_2022_7621_MOESM3_ESM.tif
Supplementary figure 3 Comparisons of predictive performance for hepatic decompensation with AUROC curves between LSM/FIB-4 at different time points (a) baseline LSM and baseline FIB-4 (b) SVR LSM and SVR FIB-4 (c) baseline LSM and SVR LSM (TIF 135 kb)
10620_2022_7621_MOESM4_ESM.tif
Supplementary figure 4 The cumulative hepatic decompensation incidences stratified by baseline LSM and SVR LSM (TIF 104 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, YC., Cheng, YT., Chen, YC. et al. Comparing Predictability of Non-invasive Tools for Hepatocellular Carcinoma in Treated Chronic Hepatitis C Patients. Dig Dis Sci 68, 323–332 (2023). https://doi.org/10.1007/s10620-022-07621-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-022-07621-6