Abstract
Background
The hyper-accumulation of extracellular matrix (ECM) is the leading cause of hepatic fibrosis, and TGF-β-induced activation of hepatic stellate cells (HSCs) is the central event of hepatic fibrosis pathogenesis. The deregulation and dysfunction of miRNAs in hepatic fibrosis have been reported previously.
Aims
To identify miRNA(s) playing a role in HSC activation and the underlying mechanism.
Methods
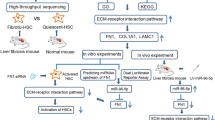
We analyzed online microarray expression datasets from Gene Expression Omnibus (GEO) for differentially expressed miRNAs in hepatic fibrosis-related disease liver tissues, examined the specific effects of the candidate miRNA on TGF-β-induced HSC activation, and screened for the targets of the candidate miRNA in the TGF-β/SMAD signaling. Then, the predicted miRNA-mRNA binding, the specific effects of the target mRNA, and the dynamic effects of miRNA and mRNA on TGF-β-induced HSC activation were investigated.
Results
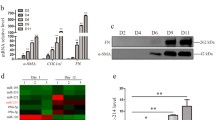
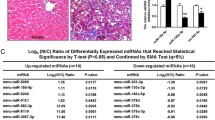
The miR-503 expression was upregulated in TGF-β-activated HSCs. miR-503 overexpression enhanced, while miR-503 inhibition attenuated TGF-β-induced HSC proliferation and ECM accumulation in HSCs. miR-503 targeted SMAD7 to inhibit SMAD7 expression. SMAD7 knockdown also aggravated TGF-β-induced HSC proliferation and ECM accumulation in HSCs. The effects of miR-503 overexpression on TGF-β-induced HSC activation were partially reversed by SMAD7 overexpression. In CCl4-induced hepatic fibrosis model in rats, miR-503 overexpression aggravated, whereas SMAD7 overexpression improved CCl4-induced fibrotic changes in rats’ liver tissues. The effects of miR-503 overexpression on CCl4-induced fibrotic changes were partially reversed by SMAD7 overexpression.
Conclusion
miR-503 acts on HSC activation and hepatic fibrosis through SMAD7. The miR-503/SMAD7 axis enhances HSC activation and hepatic fibrosis through the TGF-β/SMAD pathway.






Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
References
Jiao J, Friedman SL, Aloman C. Hepatic fibrosis. Curr Opin Gastroenterol. 2009;25:223–229.
Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218.
Ellis EL, Mann DA. Clinical evidence for the regression of liver fibrosis. J Hepatol. 2012;56:1171–1180.
Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology. 2015;61:1066–1079.
Mederacke I, Hsu CC, Troeger JS, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823.
Puche JE, Saiman Y, Friedman SL. Hepatic stellate cells and liver fibrosis. Compr Physiol. 2013;3:1473–1492.
Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015;64:830–841.
Mann DA, Marra F. Fibrogenic signalling in hepatic stellate cells. J Hepatol. 2010;52:949–950.
Hellerbrand C, Stefanovic B, Giordano F, Burchardt ER, Brenner DA. The role of TGFbeta1 in initiating hepatic stellate cell activation in vivo. J Hepatol. 1999;30:77–87.
Breitkopf K, Godoy P, Ciuclan L, Singer MV, Dooley S. TGF-beta/Smad signaling in the injured liver. Z Gastroenterol. 2006;44:57–66.
Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172.
Xu F, Liu C, Zhou D, Zhang L. TGF-beta/SMAD pathway and its regulation in hepatic fibrosis. J Histochem Cytochem. 2016;64:157–167.
Szabo G, Bala S. MicroRNAs in liver disease. Nat Rev Gastroenterol Hepatol. 2013;10:542–552.
Eulalio A, Huntzinger E, Izaurralde E. Getting to the root of miRNA-mediated gene silencing. Cell. 2008;132:9–14.
Spizzo R, Rushworth D, Guerrero M, Calin GA. RNA inhibition, microRNAs, and new therapeutic agents for cancer treatment. Clin Lymphoma Myeloma. 2009;9:S313–S318.
Dai W, Zhao J, Tang N, et al. MicroRNA-155 attenuates activation of hepatic stellate cell by simultaneously preventing EMT process and ERK1 signalling pathway. Liver Int. 2015;35:1234–1243.
Bala S, Csak T, Saha B, et al. The pro-inflammatory effects of miR-155 promote liver fibrosis and alcohol-induced steatohepatitis. J Hepatol. 2016;64:1378–1387.
Kweon SM, Chi F, Higashiyama R, Lai K, Tsukamoto H. Wnt pathway stabilizes MeCP2 protein to repress PPAR-gamma in activation of hepatic stellate cells. PLoS ONE. 2016;11:e0156111.
Cai Y, Huang G, Ma L, et al. Smurf2, an E3 ubiquitin ligase, interacts with PDE4B and attenuates liver fibrosis through miR-132 mediated CTGF inhibition. Biochim Biophys Acta Mol Cell Res. 2018;1865:297–308.
Wu K, Ye C, Lin L, et al. Inhibiting miR-21 attenuates experimental hepatic fibrosis by suppressing both the ERK1 pathway in HSC and hepatocyte EMT. Clin Sci (Lond). 2016;130:1469–1480.
Caviglia JM, Yan J, Jang MK, et al. MicroRNA-21 and Dicer are dispensable for hepatic stellate cell activation and the development of liver fibrosis. Hepatology. 2018;67:2414–2429.
Ma L, Yang X, Wei R, et al. MicroRNA-214 promotes hepatic stellate cell activation and liver fibrosis by suppressing Sufu expression. Cell Death Dis. 2018;9:718.
Ge S, Xiong Y, Wu X, et al. Role of growth factor receptor-bound 2 in CCl4-induced hepatic fibrosis. Biomed Pharmacother. 2017;92:942–951.
Ge S, Zhang L, Xie J, et al. MicroRNA-146b regulates hepatic stellate cell activation via targeting of KLF4. Ann Hepatol. 2016;15:918–928.
Yang JJ, Liu LP, Tao H, et al. MeCP2 silencing of LncRNA H19 controls hepatic stellate cell proliferation by targeting IGF1R. Toxicology. 2016;359–360:39–46.
He H, Dai J, Feng J et al. FBXO31 modulates activation of hepatic stellate cells and liver fibrogenesis by promoting ubiquitination of Smad7. J Cell Biochem. 2019. https://doi.org/10.1002/jcb.29528.
Ge S, Xie J, Liu F, He J, He J. MicroRNA-19b reduces hepatic stellate cell proliferation by targeting GRB2 in hepatic fibrosis models in vivo and in vitro as part of the inhibitory effect of estradiol. J Cell Biochem. 2015;116:2455–2464.
Zhu H, Li Y, Qu S, et al. MicroRNA expression abnormalities in limited cutaneous scleroderma and diffuse cutaneous scleroderma. J Clin Immunol. 2012;32:514–522.
Zhou Y, Deng L, Zhao D, et al. MicroRNA-503 promotes angiotensin II-induced cardiac fibrosis by targeting Apelin-13. J Cell Mol Med. 2016;20:495–505.
Mann C, Kaistha BP, Kacik M, Stiewe T, Hoyer J. Downregulation of miR-503 in activated kidney fibroblasts disinhibits KCNN4 in an in vitro model of kidney fibrosis. Kidney Blood Press Res. 2019;44:113–122.
Yan W, Wu Q, Yao W, et al. MiR-503 modulates epithelial-mesenchymal transition in silica-induced pulmonary fibrosis by targeting PI3K p85 and is sponged by lncRNA MALAT1. Sci Rep. 2017;7:11313.
Ren H, Li Y, Chen Y, Wang L. Endostatin attenuates PDGF-BB- or TGF-beta1-induced HSCs activation via suppressing RhoA/ROCK1 signal pathways. Drug Des Devel Ther. 2019;13:285–290.
Budi EH, Duan D, Derynck R. Transforming growth factor-beta receptors and smads: regulatory complexity and functional versatility. Trends Cell Biol. 2017;27:658–672.
Son G, Hines IN, Lindquist J, Schrum LW, Rippe RA. Inhibition of phosphatidylinositol 3-kinase signaling in hepatic stellate cells blocks the progression of hepatic fibrosis. Hepatology. 2009;50:1512–1523.
Yu F, Chen B, Fan X, Li G, Dong P, Zheng J. Epigenetically-regulated microRNA-9-5p suppresses the activation of hepatic stellate cells via TGFBR1 and TGFBR2. Cell Physiol Biochem. 2017;43:2242–2252.
Feili X, Wu S, Ye W, Tu J, Lou L. MicroRNA-34a-5p inhibits liver fibrosis by regulating TGF-beta1/Smad3 pathway in hepatic stellate cells. Cell Biol Int. 2018;42:1370–1376.
El-Wakeel SA, Rahmo RM, El-Abhar HS. Anti-fibrotic impact of Carvedilol in a CCl-4 model of liver fibrosis via serum microRNA-200a/SMAD7 enhancement to bridle TGF-beta1/EMT track. Sci Rep. 2018;8:14327.
Dooley S, Hamzavi J, Ciuclan L, et al. Hepatocyte-specific Smad7 expression attenuates TGF-beta-mediated fibrogenesis and protects against liver damage. Gastroenterology. 2008;135:642–659.
Hamzavi J, Ehnert S, Godoy P, et al. Disruption of the Smad7 gene enhances CCI4-dependent liver damage and fibrogenesis in mice. J Cell Mol Med. 2008;12:2130–2144.
Bian EB, Huang C, Wang H, et al. Repression of Smad7 mediated by DNMT1 determines hepatic stellate cell activation and liver fibrosis in rats. Toxicol Lett. 2014;224:175–185.
Trivedi P, Mowat AP. Carbon tetrachloride-induced hepatic fibrosis and cirrhosis in the developing rat: an experimental model of cirrhosis in childhood. Br J Exp Pathol. 1983;64:25–33.
Funding
This work was supported by the Guiding Projects of Hengyang City, 2018 (S2018F9031021273).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xie, X., Dou, CY., Zhou, Y. et al. MicroRNA-503 Targets Mothers Against Decapentaplegic Homolog 7 Enhancing Hepatic Stellate Cell Activation and Hepatic Fibrosis. Dig Dis Sci 66, 1928–1939 (2021). https://doi.org/10.1007/s10620-020-06460-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06460-7