Abstract
Background
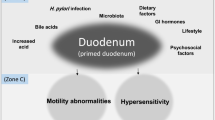
An altered gastrointestinal barrier function is reportedly associated with the pathogenesis of functional dyspepsia (FD); however, the pathogenesis of FD has not yet been fully elucidated.
Aims
The objective of the present study was to determine whether the mucosal barrier function is impaired in patients with FD and to investigate the mechanisms underlying FD.
Methods
The present study included patients with FD (FD group, n = 24), non-FD patients with abdominal symptoms (symptomatic control group, n = 14), and patients with no abdominal symptoms (asymptomatic control group, n = 20). The groups were compared regarding the mucosal electrical impedance (MI) values of the stomach and duodenum, which were measured using a tissue conductance meter during esophagogastroduodenoscopy.
Results
There were no significant differences between the three groups in the MI of the stomach. In contrast, the duodenal MI of the FD group (17.8 ± 4.3 Ω) was significantly lower than those of the symptomatic control group (27.2 ± 6.4 Ω, p < 0.0001) and asymptomatic control group (23.0 ± 7.4 Ω, p = 0.016). The expression of zonula occludens-1 (ZO-1) was significantly lower in the FD group than in the symptomatic control group (p = 0.011), where ZO-1 was positively correlated with the duodenal MI (β = 0.513, p = 0.017). The interleukin (IL)-1β expression was significantly higher in the FD group than in the symptomatic control group (p = 0.041), where IL-1β was inversely correlated with the duodenal MI (β = − 0.600, p = 0.004).
Conclusions
The mucosal barrier function of the duodenum was altered in patients with FD. Both a decreased ZO-1 and increased IL-1β may play a role in the pathogenesis of FD.




Similar content being viewed by others
References
Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–1479.
El-Serag HB, Talley NJ. The prevalence and clinical course of functional dyspepsia. Aliment Pharmacol Ther. 2004;19:643–654.
Suzuki H. The application of the Rome IV criteria to functional esophagogastroduodenal disorders in Asia. J Neurogastroenterol Motil. 2017;23:325–333.
Halling K, Kulich K, Carlsson J, Wiklund I. An international comparison of the burden of illness in patients with dyspepsia. Dig Dis. 2008;26:264–273.
Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346–1352.
Lunding JA, Tefera S, Gilja OH, et al. Rapid initial gastric emptying and hypersensitivity to gastric filling in functional dyspepsia: effects of duodenal lipids. Scand J Gastroenterol. 2006;41:1028–1036.
Shindo T, Futagami S, Hiratsuka T, et al. Comparison of gastric emptying and plasma ghrelin levels in patients with functional dyspepsia and non-erosive reflux disease. Digestion. 2009;79:65–72.
Boeckxstaens GE, Hirsch DP, Kuiken SD, Heisterkamp SH, Tytgat GN. The proximal stomach and postprandial symptoms in functional dyspeptics. Am J Gastroenterol. 2002;97:40–48.
Tack J, Caenepeel P, Fischler B, Piessevaux H, Janssens J. Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology. 2001;121:526–535.
Bratten J, Jones MP. Prolonged recording of duodenal acid exposure in patients with functional dyspepsia and controls using a radiotelemetry pH monitoring system. J Clin Gastroenterol. 2009;43:527–533.
Feinle C, Meier O, Otto B, D’Amato M, Fried M. Role of duodenal lipid and cholecystokinin A receptors in the pathophysiology of functional dyspepsia. Gut. 2001;48:347–355.
Hammer J, Fuhrer M. Clinical characteristics of functional dyspepsia depending on chemosensitivity to capsaicin. Neurogastroenterol Motil. 2017;29:1–12.
Kindt S, Tertychnyy A, de Hertogh G, Geboes K, Tack J. Intestinal immune activation in presumed post-infectious functional dyspepsia. Neurogastroenterol Motil. 2009;21:832–856.
Talley NJ, Walker MM, Aro P, et al. Non-ulcer dyspepsia and duodenal eosinophilia: an adult endoscopic population-based case–control study. Clin Gastroenterol Hepatol. 2007;5:1175–1183.
Vanheel H, Vicario M, Vanuytsel T, et al. Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia. Gut. 2014;63:262–271.
Ishigami H, Matsumura T, Kasamatsu S, et al. Endoscopy-guided evaluation of duodenal mucosal permeability in functional dyspepsia. Clin Transl Gastroenterol. 2017;8:e83.
Koizumi H, Suzuki H, Ohbuchi T, Kitamura T, Hashida K, Nakamura M. Increased permeability of the epithelium of middle ear cholesteatoma. Clin Otolaryngol. 2015;40:106–114.
Yamamoto T, Yamamoto Y. Electrical properties of the epidermal stratum corneum. Med Biol Eng. 1976;14:151–158.
Lawrence JN. Electrical resistance and tritiated water permeability as indicators of barrier integrity of in vitro human skin. Toxicol In Vitro. 1997;11:241–249.
Martinsen OG, Grimnes S, Nilsen SH. Water sorption and electrical properties of a human nail. Skin Res Technol. 2008;14:142–146.
Suzuki H, Koizumi H, Ikezaki S, et al. Electrical impedance and expression of tight junction components of the nasal turbinate and polyp. ORL J Otorhinolaryngol Relat Spec. 2016;78:16–25.
Farre R, Blondeau K, Clement D, et al. Evaluation of oesophageal mucosa integrity by the intraluminal impedance technique. Gut. 2011;60:885–892.
Iboshi Y, Nakamura K, Ihara E, et al. Multigene analysis unveils distinctive expression profiles of helper T-cell-related genes in the intestinal mucosa that discriminate between ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis. 2014;20:967–977.
Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17:564–580.
Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60.
Thaiss CA, Levy M, Grosheva I, et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science. 2018;359:1376–1383.
Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766.
Umeda K, Ikenouchi J, Katahira-Tayama S, et al. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754.
Montalto M, Cuoco L, Ricci R, Maggiano N, Vecchio FM, Gasbarrini G. Immunohistochemical analysis of ZO-1 in the duodenal mucosa of patients with untreated and treated celiac disease. Digestion. 2002;65:227–233.
Pizzuti D, Bortolami M, Mazzon E, et al. Transcriptional downregulation of tight junction protein ZO-1 in active coeliac disease is reversed after a gluten-free diet. Dig Liver Dis. 2004;36:337–341.
Bueno L, Fioramonti J. Protease-activated receptor 2 and gut permeability: a review. Neurogastroenterol Motil. 2008;20:580–587.
Jacob C, Yang PC, Darmoul D, et al. Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and beta-arrestins. J Biol Chem. 2005;280:31936–31948.
Hirano M, Hirano K. Myosin di-phosphorylation and peripheral actin bundle formation as initial events during endothelial barrier disruption. Sci Rep. 2016;6:20989.
Liebregts T, Adam B, Bredack C, et al. Small bowel homing T cells are associated with symptoms and delayed gastric emptying in functional dyspepsia. Am J Gastroenterol. 2011;106:1089–1098.
Al-Sadi RM, Ma TY. IL-1β causes an increase in intestinal epithelial tight junction permeability. J Immunol. 2007;178:4641–4649.
Cui HS, Hayasaka S, Zhang XY, Hayasaka Y, Chi ZL, Zheng LS. Effect of berberine on barrier function in a human retinal pigment epithelial cell line. Jpn J Ophthalmol. 2007;51:64–67.
Kimura K, Teranishi S, Nishida T. Interleukin-1β-induced disruption of barrier function in cultured human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2009;50:597–603.
Tanaka H, Fukuda K, Ishida W, Harada Y, Sumi T, Fukushima A. Rebamipide increases barrier function and attenuates TNFα-induced barrier disruption and cytokine expression in human corneal epithelial cells. Br J Ophthalmol. 2013;97:912–916.
Esnault S, Kelly EA, Nettenstrom LM, Cook EB, Seroogy CM, Jarjour NN. Human eosinophils release IL-1β and increase expression of IL-17A in activated CD4+ T lymphocytes. Clin Exp Allergy. 2012;42:1756–1764.
Futagami S, Shindo T, Kawagoe T, et al. Migration of eosinophils and CCR2-/CD68-double positive cells into the duodenal mucosa of patients with postinfectious functional dyspepsia. Am J Gastroenterol. 2010;105:1835–1842.
Walker MM, Salehian SS, Murray CE, et al. Implications of eosinophilia in the normal duodenal biopsy—an association with allergy and functional dyspepsia. Aliment Pharmacol Ther. 2010;31:1229–1236.
Saritas Yuksel E, Higginbotham T, Slaughter JC, et al. Use of direct, endoscopic-guided measurements of mucosal impedance in diagnosis of gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2012;10:1110–1116.
van Rhijn BD, Weijenborg PW, Verheij J, et al. Proton pump inhibitors partially restore mucosal integrity in patients with proton pump inhibitor-responsive esophageal eosinophilia but not eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2014;12:1815–1823.
Li X, Chen H, Lu H, et al. The study on the role of inflammatory cells and mediators in post-infectious functional dyspepsia. Scand J Gastroenterol. 2010;45:573–581.
Acknowledgment
We appreciate the technical assistance we received from the Research Support Center, Research Center for Human Disease Modeling, Kyushu University Graduate School of Medical Sciences. We thank Kelly Zammit, BVSc, from Edanz Editing (www.edanzediting.com/ac), for editing a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
KK and HO proposed the study. KK, EI, and YM performed the research. KK and YM collected and analyzed the data. TS, MF, and YO performed the histological diagnosis. KK and EI wrote the manuscript. YO supervised the study. All authors contributed to the interpretation of the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.

10620_2019_5470_MOESM1_ESM.jpg
Supplemental Figure 1. Correlation between the esophageal mucosal electrical impedance (MI) measured using the 24-h multichannel intraluminal pH/impedance test (MII-pH) versus the tissue conductance meter. The esophageal MI was measured in 14 patients (from either the functional dyspepsia group or the symptomatic control group) using both the MII-pH and the tissue conductance meter. The correlation between the MI values obtained using the different devices was calculated by performing Pearson’s correlation analysis. (JPEG 173 kb)
Rights and permissions
About this article
Cite this article
Komori, K., Ihara, E., Minoda, Y. et al. The Altered Mucosal Barrier Function in the Duodenum Plays a Role in the Pathogenesis of Functional Dyspepsia. Dig Dis Sci 64, 3228–3239 (2019). https://doi.org/10.1007/s10620-019-5470-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-5470-8