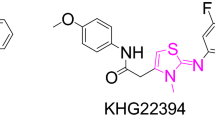
An effective one-pot three-component protocol for the synthesis of polysubstituted thiazole derivatives is reported. The reaction proceeds between arylglyoxals, thiosemicarbazones, and acetylacetone or ethyl acetoacetate in ethanol as solvent and in the presence of acetic acid as catalyst.



Similar content being viewed by others
References
Ganesh, T.; Schilling, J. K.; Palakodety, R. K.; Ravindra, R.; Shanker, N.; Bane, S.; Kingston, D. G. I. Tetrahedron 2003, 59, 9979.
Nicolaou, K. C.; Ritzen, A.; Namoto, K. Chem. Commun. 2001, 1523.
Hu, D.-J.; Liu, S.-F.; Huang, T.-H.; Tu, H.-Y.; Zhang, A.-D. Molecules 2009, 14, 1288.
Van Muijlwijk-Koezen, J. E.; Timmerman, H.; Vollinga, R. C.; Frijtag von Drabbe Künzel, J.; de Groote, M.; Visser, S.; Ijzerman, A. P. J. Med. Chem. 2001, 44, 749.
Mahboobi, S.; Sellmer, A.; Höcher, H.; Eichhorn, E.; Bar, T.; Schmidt, M.; Maier, T.; Stadlwieser, J. F.; Beckers, T. L. J. Med. Chem. 2006, 49, 5769.
Sultanova, R. M.; Lobov, A. N.; Shumadalova, A. V.; Meshcheryakova, S. A.; Zileeva, Z. R.; Khusnutdinova, N. S.; Vakhitov, V. A.; Vakhitova, Y. V. Nat. Prod. Res. 2021, 35, 1340.
Tsuji, K.; Ishikawa, H. Bioorg. Med. Chem. Lett. 1994, 4, 1601.
Haviv, F.; Ratajczyk, J. D.; DeNet, R. W.; Kerdesky, F. A.; Walters, R. L.; Schmidt, S. P.; Holms, J. H.; Young, P. R.; Carter, G. W. J. Med. Chem. 1988, 31, 1719.
Miwatashi, S.; Arikawa, Y.; Kotani, E.; Miyamoto, M.; Naruo, K. I.; Kimura, H.; Yanaka, T.; Asahi, S.; Ohkawa, S. J. Med. Chem. 2005, 48, 5966.
Papadopoulou, C.; Geronikaki, A.; Hadjipavlou-Litina, D. Farmaco 2005, 60, 969.
Patt, W. C.; Hamilton, H. W.; Taylor, M. D.; Ryan, M. J.; Taylor, D. G. J.; Connolly, C. J. C.; Doherty, A. M.; Klutchko, S. R.; Sircar, I.; Steinbaugh, B. A.; Batley, B. L.; Painchaud, C. A.; Rapundalo, S. T.; Michniewicz, B. M.; Olson, S. C. J. J. Med. Chem. 1992, 35, 2562.
Tsuruni, Y.; Ueda, H.; Hayashi, K.; Takase, S.; Nishikawa, M.; Kiyoto, S.; Okuhara, M. J. Antibiotics 1995, 48, 1066.
Bell, F. W.; Cantrell, A. S.; Hoegberg, M.; Jaskunas, S. R.; Johansson, N. G.; Jordan, C. L.; Kinnick, M. D.; Lind, P.; Morin, J. M., Jr.; Noveen, R.; Oberg, B.; Palkowitz, J. A.; Parrish, C. A.; Pranc, P.; Sahlberg, C.; Ternansky, R. J.; Vasileft, R. T.; Vrang, L.; West, S. J.; Zhang, H.; Zhou, X. Y. J. Med. Chem. 1995, 38, 4929.
Kumar, Y.; Green, R.; Borysko, K. Z.; Wise, D. S.; Wotring, L. L.; Townsend, L. B. J. Med. Chem. 1993, 36, 3843.
Ei-Subbagh, H. I.; Al-Obaid, A. M. Eur. J. Med. Chem. 1996, 31, 1017.
Pereira, R.; Gaudon, C.; Iglesias, B.; Germain, P.; Gronemeyer, H.; de Lera, A. R. Bioorg. Med. Chem. Lett. 2006, 16, 49.
Potewar, T. M.; Ingale, S. A.; Srinivasan, K. V. Tetrahedron 2007, 63, 11066.
Narender, M.; Reddy, M. S.; Sridhar, R.; Nageswar, Y. V. D.; Rao, K. R. Tetrahedron Lett. 2005, 46, 5953.
Das, B.; Reddy, V. S.; Ramu, R. J. Mol. Catal. A: Chem. 2006, 252, 235.
Liu, X. L.; Wang, Q. Y.; Sheng, S. R.; Xu, C.; Cai, M. Z. Synth. Commun. 2008, 38, 3338.
Wei, S.; Weiß, K. M.; Tsogoeva, S. B. Synthesis 2012, 44, 3441.
Weiß, K. M.; Wei, S.; Tsogoeva, S. B. Org. Biomol. Chem. 2011, 9, 3457.
Gao, X.; Pan, Y. M.; Lin, M.; Chen, L.; Zhan, Z. P. Org. Biomol. Chem. 2010, 8, 3259.
Al-Hourani, B. J.; Banert, K.; Gomaa, N.; Vrobel, K. Tetrahedron 2008, 64, 5590.
Sheldrake, P. W.; Matteucci, M.; McDonald, E. Synlett 2006, 460.
Zhang, G.; Chen, B.; Guo, X.; Guo, S.; Yu, Y. Adv. Synth. Catal. 2015, 357, 1065.
Chen, B.; Guo, S.; Guo, X.; Zhang, G.; Yu, Y. Org. Lett. 2015, 17, 4698.
Karamthulla, S.; Pal, S.; Khan, M. N.; Choudhury, L. H. RSC Adv. 2014, 4, 37889.
Saroha, M.; Khurana, J. M. New J. Chem. 2019, 43, 8644.
Mousavizadeh, F.; Talebizadeh, M.; Anary-Abbasinejad, M. Tetrahedron Lett. 2018, 59, 2970.
Anary-Abbasinejad, M.; Nezhad-Shahrokhabadi, F.; Mohammadi, M. Mol. Diversity 2020, 24, 1205.
Dehghanzadeh, F.; Shahrokhabadi, F.; Anary-Abbasinejad, M. ARKIVOC 2019, (v), 133.
Riley, H. A.; Gray, A. R. Organic Synthesis; Wiley: New York, 1943, vol. 2, p. 509.
Khalid, M.; Jawaria, R.; Khan, M. U.; Braga, A. A. C.; Shafiq, Z.; Imran, M.; Zafar, H. M. A.; Irfan, A. ACS Omega 2021, 6, 16058.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2023, 59(9/10), 709–712
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Latifi, M., Anary-Abbasinejad, M. & Mohammadi, M. A simple route for the synthesis of substituted thiazole derivatives by multicomponent reaction between arylglyoxals, acetylacetone or ethyl acetoacetate, and thiosemicarbazones. Chem Heterocycl Comp 59, 709–712 (2023). https://doi.org/10.1007/s10593-023-03258-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-023-03258-z