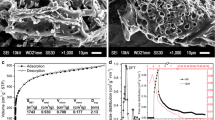
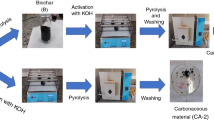
Abstract
In this study, sugarcane bagasse was used to prepare urea phosphate activated carbons (UPACs) using a novel activator urea phosphate (UP) at three different temperatures (300 ℃, 550 ℃, and 800 ℃) to analyze the reaction mechanism during the pyrolysis process by Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy (Raman), elemental analysis, and thermogravimetric Fourier transform infrared spectroscopy-mass spectrometry (TG-FTIR-MS), to deduce the reaction mechanism of UP activation. Below 300 °C, the functional groups on the surface of sugarcane bagasse fibers undergo hydroxyl dehydration and oxidation reactions, and molecular chains were broken to produce H2O, CH4, CO2, H2, HCHO, and NH3 small molecule gas products. At 300–800 °C was the main temperature range for activation reactions, and the molecular structure gradually formed an ordered carbon network structure. Nitrogen-containing compounds were gradually transformed into graphite N and oxidized N as temperature increased and functional groups containing phosphorus underwent decomposition. Above 800 °C, the pyrolysis was basically complete. Phosphorus compounds were completely decomposed, with fewer macromolecular products and gaseous products being mainly H2O, CO2, CO, and HCHO.
Graphical Abstract









Similar content being viewed by others
Data availability
All relevant data are within the manuscript, which is available from the corresponding author upon request.
References
Ayinla RT, Dennis JO, Zaid HM, Sanusi YK, Usman F, Adebayo L (2019) A review of technical advances of recent palm bio-waste conversion to activated carbon for energy storage. J Clean Prod 229:1427–1442. https://doi.org/10.1016/j.jclepro.2019.04.116
Cai H, Liu J, Xie W, Kuo J, Buyukada M, Evrendilek F (2019) Pyrolytic kinetics, reaction mechanisms and products of waste tea via TG-FTIR and Py-GC/MS. Energ Convers Manage 184:436–447. https://doi.org/10.1016/j.enconman.2019.01.031
Chen M, Bao C, Hu D, Jin X, Huang Q (2019) Facile and low-cost fabrication of ZnO/biochar nanocomposites from jute fibers for efficient and stable photodegradation of methylene blue dye. J Anal Appl Pyrol 139:319–332. https://doi.org/10.1016/j.jaap.2019.03.009
Ding Y, Ezekoye OA, Lu S, Wang C (2016) Thermal degradation of beech wood with thermogravimetry/fourier transform infrared analysis. Energ Convers Manage 120:370–377. https://doi.org/10.1016/j.enconman.2016.05.007
Ferdous D, Dalai AK, Bej SK, Thring RW, Bakhshi N (2001) Production of H2 and medium btu gas via pyrolysis of lignins in a fixed-bed reactor. Fuel Process Technol 70(1):9–26. https://doi.org/10.1016/S0378-3820(00)00147-8
Guo Z, Zhang X, Kang Y, Zhang J (2017) Biomass-derived carbon sorbents for Cd (II) removal: activation and adsorption mechanism. ACS Sustain Chem Eng 5(5):4103–4109. https://doi.org/10.1021/acssuschemeng.7b00061
Guo Y, Tan C, Sun J, Li W, Zhang J, Zhao C (2020) Porous activated carbons derived from waste sugarcane bagasse for CO2 adsorption. Chem Eng J 381:122736. https://doi.org/10.1016/j.cej.2019.122736
Huang R, Liu HY, Zhang BS, Sun X, Liang C, Su D, Zong B, Rong J (2015a) Phosphate-modified carbon nanotubes in the oxidative dehydrogenation of isopentanes. Chemsuschem 7(12):3476–3482. https://doi.org/10.1002/cssc.201402457
Huang Y, Ma E, Zhao G (2015b) Thermal and structure analysis on reaction mechanisms during the preparation of activated carbon fibers by KOH activation from liquefied wood-based fibers. Ind Crop Prod 69:447–455. https://doi.org/10.1016/j.indcrop.2015.03.002
Huang Y, Liu Z, Zhao G (2016) Reaction process for ZnCl2 activation of phenol liquefied wood fibers. RSC Adv 6(82):78909–78917. https://doi.org/10.1039/c6ra15705j
Lauševié Z, Marinkovié S (1986) Mechanical properties and chemistry of carbonization of phenol formaldehyde resin. Carbon 24(5):575–580. https://doi.org/10.1016/0008-6223(86)90148-x
Li Y, Zhu S, Liu Q, Chen Z, Gu J, Zhu C, Lu T, Zhang D, Ma J (2013) N-doped porous carbon with magnetic particles formed in situ for enhanced Cr (VI) removal. Water Res 47(12):4188–4197. https://doi.org/10.1016/j.watres.2012.10.056
Li Y, Zhao H, Chen S, Bao S, Xing F, Jiang B (2021) Phosphorus-doped activated carbon catalyst for n-hexane dehydroaromatization reaction. Catal Commun 156:106318. https://doi.org/10.1016/j.catcom.2021.106318
Liu Q, Lv C, Yang Y, He F, Ling L (2005) Study on the pyrolysis of wood-derived rayon fiber by thermogravimetry–mass spectrometry. J Mol Struct 733(13):193–202. https://doi.org/10.1016/j.molstruc.2004.01.016
Luo X, Cai Y, Liu L, Zeng J (2019) Cr (VI) adsorption performance and mechanism of an effective activated carbon prepared from sugarcane bagasse with a one-step pyrolysis and ZnCl2 activation Method. Cellulose 26(8):4921–4934. https://doi.org/10.1007/s10570-019-02418-9
Ma Z, Chen D, Gu J, Bao B, Zhang Q (2015) Determination of pyrolysis characteristics and kinetics of palm kernel shell using TGA–FTIR and model-free integral methods. Energ Convers Manage 89:251–259. https://doi.org/10.1016/j.enconman.2014.09.074
McCullough JF, Sheridan RC, Frederick L (1978) Pyrolysis of urea phosphate. J Agric Food Chem 26(3):670–675. https://doi.org/10.1021/jf60217a060
Moniruzzaman M, Ono T (2013) Separation and characterization of cellulose fibers from cypress wood treated with ionic liquid prior to laccase treatment. Bioresour Technol 127:132–137. https://doi.org/10.1016/j.biortech.2012.09.113
Otowa T, Tanibata R, Itoh M (1993) Production and adsorption characteristics of MAXSORB: high-surface-area active carbon. Gas Sep and Purif 7(4):241–245. https://doi.org/10.1016/0950-4214(93)80024-q
Prauchner MJ, Sapag K, Rodríguez-Reinoso F (2016) Tailoring biomass-based activated carbon for CH4 storage by combining chemical activation with H3PO4 or ZnCl2 and physical activation with CO2. Carbon 110:138–147. https://doi.org/10.1016/j.carbon.2016.08.092
Rodrigues CSD, Soares OSGP, Pinho MT, Pereira MFR, Madeira LM (2017) P-Nitrophenol degradation by heterogeneous fenton’s oxidation over activated carbon-based catalysts. Appl Catal B Environ 219:109–122. https://doi.org/10.1016/j.apcatb.2017.07.045
Rodríguez-Machín L, Arteaga-Pérez LE, Pérez-Bermúdez RA, Casas-Ledón Y, Prins W, Ronsse F (2018) Effect of citric acid leaching on the demineralization and thermal degradation behavior of sugarcane trash and bagasse. Biomass and Bioenerg 108:371–380. https://doi.org/10.1016/j.biombioe.2017.11.001
Sayğılı H, Sayğılı GA (2019) Optimized preparation for bimodal porous carbon from lentil processing waste by microwave-assisted K2CO3 activation: spectroscopic characterization and dye decolorization activity. J Clean Prod 226:968–976. https://doi.org/10.1016/j.jclepro.2019.04.121
Shen DK, Gu S (2009) The mechanism for thermal decomposition of cellulose and its main Products. Bioresour Technol 100(24):6496–6504. https://doi.org/10.1016/j.biortech.2009.06.095
Shin S, Jang J, Yoon S-H, Mochida I (1997) A study on the effect of heat treatment on functional groups of pitch based activated carbon fiber using FTIR. Carbon 35(12):1739–1743. https://doi.org/10.1016/s0008-6223(97)00132-2
Singh G, Kim IY, Lakhi KS, Srivastava P, Naidu P, Vinu A (2017) Single step synthesis of activated bio-carbons with a high surface area and their excellent CO2 adsorption capacity. Carbon 116:448–455. https://doi.org/10.1016/j.carbon.2017.02.015
Singh G, Lakhi KS, Sil S, Bhosale SV, Kim I, Albahily K, Vinu A (2019) Biomass derived porous carbon for CO2 capture. Carbon 148:164–186. https://doi.org/10.1016/j.carbon.2019.03.050
Wang G, Zhang L, Zhang J (2012) A review of electrode materials for electrochemical supercapacitors. Chem Soc Rev 41(2):797–828. https://doi.org/10.1039/c1cs15060j
Wang Q, Lai Z, Luo C, Zhang J, Cao X, Liu J, Mu J (2021) Honeycomb-like activated carbon with microporous nanosheets structure prepared from waste biomass cork for highly efficient dye wastewater treatment. J Hazard 416:125896. https://doi.org/10.1016/j.jhazmat.2021.125896
Wu T, Wang G, Dong Q, Zhan F, Zhang X, Li S, Qiao H, Qiu J (2017) Starch derived porous carbon nanosheets for high-performance photovoltaic capacitive deionization. Environ Sci Technol 51(16):9244–9251. https://doi.org/10.1021/acs.est.7b01629
Xia Y, Zuo H, Lv J, Wei S, Yao Y, Liu Z, Lin Q, Huang Y (2023) Preparation of multi-layered microcapsule-shaped activated biomass carbon with ultrahigh surface area from bamboo parenchyma cells for energy storage and cationic dyes removal. J Clean Prod 25:136517. https://doi.org/10.1016/j.jclepro.2023.136517
Yan B, Jiao L, Li J, Zhu X, Ahmed S, Chen G (2021) Investigation on microwave torrefaction: parametric influence, TG-MS-FTIR analysis, and gasification performance. Energy 220:119794. https://doi.org/10.1016/j.energy.2021.119794
Yang H, Yan R, Chen H, Lee DH, Zheng C (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86:1781–1788. https://doi.org/10.1016/j.fuel.2006.12.013
Yang CS, Jang YS, Jeong HK (2014) Bamboo-based activated carbon for supercapacitor applications. Curr Appl Phys 14(12):1616–1620. https://doi.org/10.1016/j.cap.2014.09.021
Zhang N, Shen Y (2019) One-step pyrolysis of lignin and polyvinyl chloride for synthesis of porous carbon and its application for toluene Sorption. Bioresour Technol 284:325–332. https://doi.org/10.1016/j.biortech.2019.03.149
Zhao Y, Yan N, Feng MW (2013) Thermal degradation characteristics of phenol–formaldehyde resins derived from beetle infested pine barks. Thermochim Acta 555:46–52. https://doi.org/10.1016/j.tca.2012.12.002
Zhao J, Yu L, Ma H, Zhou F, Yang K, Wu G (2020) Corn Stalk-based activated carbon synthesized by a novel activation method for high-performance adsorption of hexavalent chromium in aqueous solutions. J Colloid Interface Sci 578:650–659. https://doi.org/10.1016/j.jcis.2020.06.031
Zhou Q, Jiang X, Li X, Jia CQ, Jiang W (2018) Preparation of High-yield N-doped biochar from nitrogen-containing phosphate and its effective adsorption for Toluene. RSC Adv 8(53):30171–30179. https://doi.org/10.1039/c8ra05714a
Zhu L, Qi H, Lv M, Kong Y, Yu Y, Xu X (2012) Component analysis of extracellular polymeric substances (EPS) during aerobic sludge granulation using FTIR and 3D-EEM Technologies. Bioresour Technol 124:455–459. https://doi.org/10.1016/j.biortech.2012.08.059
Zuo H, Xia Y, Liu H, Liu Z, Huang Y (2023) Preparation of activated carbon with high nitrogen content from agro-industrial waste for efficient treatment of chromium (VI) in water. Ind Crop Prod 194:116403. https://doi.org/10.1016/j.indcrop.2023.116403
Acknowledgments
This work was supported by the Materials Center of Tsinghua University. We acknowledge Beijing Zhongkebaice Technology Service Co., Ltd. for the characterization results.
Funding
The National Natural Science Foundation of China (No. 32060322).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by H.Z. The first draft of the manuscript was written by H.Z. and all authors commented on previous versions of the manuscript. Funding, writing review, supervision and validation of the thesis is the responsibility of Z.L. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known conflict of interest or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval and consent to participate
This article does not contain any studies or investigations conducted by the authors on human participants or animals that violate ethical standards.
Consent for publication
The authors agree to publish the article under the Creative Commons Attribution Licence.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zuo, H., Liu, Z. Thermal and structural analysis of the reaction mechanisms during the preparation of activated carbon from sugarcane bagasse by urea phosphate activation. Cellulose 31, 793–808 (2024). https://doi.org/10.1007/s10570-023-05622-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05622-w