Abstract
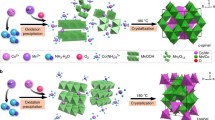
Cobaltite spinel oxides (CuCo2O4), which show electrocatalytic activity for oxygen evolution reaction (OER), are readily synthesized using a facile hydrothermal method, with a stationary ratio, uniform flower-like mesopores morphology, and high crystallinity. We have carried out first-principles calculations on the mechanism of the reaction pathway and the Gibbs free energy diagram of CuCo2O4 structures using density functional theory and purely confirmed by experimental results. This catalyst performed an outstanding OER performance with an overpotential 230 mV at 10 mA cm−2 in 1 M KOH, which was close to IrO2 with an overpotential 190 mV at 10 mA cm−2. This work provides a facile method for electrocatalytic oxygen production with enhanced conductivity and enhanced OER by replacing cobalt with copper.
Graphical Abstract







Similar content being viewed by others
References
Wu T, Sun S, Song J, Xi S, Du Y, Chen B, Sasangka WA, Liao H, Gan CL, Scherer GG, Zeng L, Wang H, Li H, Grimaud A, Xu ZJ (2019) Iron-facilitated dynamic active-site generation on spinel CoAl2O4 with self-termination of surface reconstruction for water oxidation. Nat Catal 2:763–772
Zhao D, Dai M, Zhao Y, Liu H, Liu Y, Wu X (2020) Improving electrocatalytic activities of FeCo2O4@FeCo2S4@PPy electrodes by surface/interface regulation. Nano Energy 72:104715
Li J, Zheng G (2017) One-dimensional earth-abundant nanomaterials for water-splitting electrocatalysts. Adv Sci 4:1600380
Jiao Y, Zheng Y, Jaroniec M, Qiao SZ (2015) Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem Soc Rev 44:2060–2086
Aqueel Ahmed AT, Hou B, Chavan HS, Jo Y, Cho S, Kim J, Pawar SM, Cha S, Inamdar AI, Kim H, Im H (2018) Self-assembled nanostructured CuCo2O4 for electrochemical energy storage and the oxygen evolution reaction via morphology engineering. Small 14:e1800742
Yu M, Budiyanto E, Tüysüz H (2022) Principles of water electrolysis and recent progress in cobalt-, nickel-, and iron-based oxides for the oxygen evolution reaction. Angew Chem Int Ed Engl 61:e202103824
Li Y, Wang Y, Lu J, Yang B, San X, Wu Z-S (2020) 2D intrinsically defective RuO2/graphene heterostructures as all-pH efficient oxygen evolving electrocatalysts with unprecedented activity. Nano Energy 78:105185
Chen J, Cui P, Zhao G, Rui K, Lao M, Chen Y, Zheng X, Jiang Y, Pan H, Dou SX, Sun W (2019) Low-coordinate iridium oxide confined on graphitic carbon nitride for highly efficient oxygen evolution. Angew Chem Int Ed Engl 58:12540–12544
Zou X, Zhang Y (2015) Noble metal-free hydrogen evolution catalysts for water splitting. Chem Soc Rev 44:5148–5180
Zhu C, Wang AL, Xiao W, Chao D, Zhang X, Tiep NH, Chen S, Kang J, Wang X, Ding J, Wang J, Zhang H, Fan HJ (2018) In situ grown epitaxial heterojunction exhibits high-performance electrocatalytic water splitting. Adv Mater 30:e1705516
Kou Z, Wang K, Liu Z, Zeng L, Li Z, Yang B, Lei L, Yuan C, Hou Y (2021) Recent advances in manifold exfoliated synthesis of two-dimensional non-precious metal-based nanosheet electrocatalysts for water splitting. Small Struct 3:2100153
Zheng X, Chen Y, Zheng X, Zhao G, Rui K, Li P, Xu X, Cheng Z, Dou SX, Sun W (2019) Electronic structure engineering of LiCoO2 toward enhanced oxygen electrocatalysis. Adv Energy Mater 9:1803482
Huang K, Zhao Z, Du H, Du P, Wang H, Wang R, Lin S, Wei H, Long Y, Lei M, Guo W, Wu H (2020) Rapid thermal annealing toward high-quality 2D cobalt fluoride oxide as an advanced oxygen evolution electrocatalyst. ACS Sustain Chem Eng 8:6905–6913
Huynh M, Bediako DK, Nocera DG (2014) A functionally stable manganese oxide oxygen evolution catalyst in acid. J Am Chem Soc 136:6002–6010
Hou X, Zhou H, Zhao M, Cai Y, Wei Q (2020) MoS2 nanoplates embedded in Co–N-doped carbon nanocages as efficient catalyst for HER and OER. ACS Sustain Chem Eng 8:5724–5733
Xue H, Zhang H, Fricke S, Lüther M, Yang Z, Meng A, Bremser W, Li Z (2020) Scalable and energy-efficient synthesis of CoxP for overall water splitting in alkaline media by high energy ball milling. Sustain Energy Fuels 4:1723–1729
Chen Z, Duan X, Wei W, Wang S, Ni B-J (2019) Recent advances in transition metal-based electrocatalysts for alkaline hydrogen evolution. J Mater Chem A 7:14971–15005
Luxa J, Cintl Š, Spejchalová L, Lin J-Y, Sofer Z (2020) Potential dependent electrochemical exfoliation of NiPS3 and implications for hydrogen evolution reaction. ACS Appl Energy Mater 3:11992–11999
Jin H, Gu Q, Chen B, Tang C, Zheng Y, Zhang H, Jaroniec M, Qiao SZ (2020) Molten salt-directed catalytic synthesis of 2D layered transition-metal nitrides for efficient hydrogen evolution. Chem 6:2382–2394
Urbankowski P, Anasori B, Makaryan T, Er D, Kota S, Walsh PL, Zhao M, Shenoy VB, Barsoum MW, Gogotsi Y (2016) Synthesis of two-dimensional titanium nitride Ti4N3 (MXene). Nanoscale 8:11385–11391
Lee J, Noh S, Pham ND, Shim JH (2019) Top-down synthesis of S-doped graphene nanosheets by electrochemical exfoliation of graphite: metal-free bifunctional catalysts for oxygen reduction and evolution reactions. Electrochim Acta 313:1–9
Duan J, Chen S, Jaroniec M, Qiao SZ (2015) Heteroatom-doped graphene-based materials for energy-relevant electrocatalytic processes. ACS Catal 5:5207–5234
Yuan Z, Li J, Yang M, Fang Z, Jian J, Yu D, Chen X, Dai L (2019) Ultrathin black phosphorus-on-nitrogen doped graphene for efficient overall water splitting: dual modulation roles of directional interfacial charge transfer. J Am Chem Soc 141:4972–4979
Islam MS, Kim M, Jin X, Oh SM, Lee N-S, Kim H, Hwang S-J (2018) Bifunctional 2D superlattice electrocatalysts of layered double hydroxide-transition metal dichalcogenide active for overall water splitting. ACS Energy Lett 3:952–960
Liu R, Wang Y, Liu D, Zou Y, Wang S (2017) Water-plasma-enabled exfoliation of ultrathin layered double hydroxide nanosheets with multivacancies for water oxidation. Adv Mater 29:1701546
Li F, Li J, Zhou L, Dai S (2021) Enhanced OER performance of composite Co–Fe-based MOF catalysts via a one-pot ultrasonic-assisted synthetic approach. Sustain Energy Fuels 5:1095–1102
Jayaramulu K, Masa J, Morales DM, Tomanec O, Ranc V, Petr M, Wilde P, Chen YT, Zboril R, Schuhmann W, Fischer RA (2018) Ultrathin 2D cobalt zeolite-imidazole framework nanosheets for electrocatalytic oxygen evolution. Adv Sci 5:1801029
Bai K, Fan J-C, Shi P-H, Min Y-L, Xu Q-J (2020) Directly ball milling red phosphorus and expended graphite for oxygen evolution reaction. J Power Sources 456:228003
Gusmao R, Sofer Z, Bousa D, Pumera M (2017) Pnictogen (As, Sb, Bi) nanosheets for electrochemical applications are produced by shear exfoliation using kitchen blenders. Angew Chem Int Ed Engl 56:14417–14422
Ensafi AA, Moosavifard SE, Rezaei B, Kaverlavani SK (2018) Engineering onion-like nanoporous CuCo2O4 hollow spheres derived from bimetal–organic frameworks for high-performance asymmetric supercapacitors. J Mater Chem A 6:10497–10506
Wei G, He J, Zhang W, Zhao X, Qiu S, An C (2018) Rational design of Co(II) dominant and oxygen vacancy defective CuCo2O4@CQDs hollow spheres for enhanced overall water splitting and supercapacitor performance. Inorg Chem 57:7380–7389
Song K, Ai W, Zhang Y, Zeng Y, Yu Y, Qiao H, Liu Z, Shen X, Hu X, Hu X (2021) Three-dimensional self-supported CuCo2O4 nanowires@NiO nanosheets core/shell arrays as an oxygen electrode catalyst for Li–O2 batteries. J Mater Chem A 9:3007–3017
Zhang P, He H (2020) Rational rope-like CuCo2O4 nanosheets directly on Ni foam as multifunctional electrodes for supercapacitor and oxygen evolution reaction. J Alloys Compd 826:153993
Serov A, Andersen NI, Roy AJ, Matanovic I, Artyushkova K, Atanassov P (2015) CuCo2O4 ORR/OER bi-functional catalyst: influence of synthetic approach on performance. J Electrochem Soc 162:F449–F454
Pendashteh A, Moosavifard SE, Rahmanifar MS, Wang Y, El-Kady MF, Kaner RB, Mousavi MF (2015) Highly ordered mesoporous CuCo2O4 nanowires, a promising solution for high-performance supercapacitors. Chem Mater 27:3919–3926
Cheng J, Yan H, Lu Y, Qiu K, Hou X, Xu J, Han L, Liu X, Kim J-K, Luo Y (2015) Mesoporous CuCo2O4 nanograsses as multi-functional electrodes for supercapacitors and electro-catalysts. J Mater Chem A 3:9769–9776
Liu S, Hui KS, Hui KN (2016) Flower-like copper cobaltite nanosheets on graphite paper as high-performance supercapacitor electrodes and enzymeless glucose sensors. ACS Appl Mater Interfaces 8:3258–3267
Kang W, Tang Y, Li W, Li Z, Yang X, Xu J, Lee CS (2014) Porous CuCo2O4 nanocubes wrapped by reduced graphene oxide as high-performance lithium-ion battery anodes. Nanoscale 6:6551–6556
Kaverlavani SK, Moosavifard SE, Bakouei A (2017) Designing graphene-wrapped nanoporous CuCo2O4 hollow spheres electrodes for high-performance asymmetric supercapacitors. J Mater Chem A 5:14301–14309
Kamari Kaverlavani S, Moosavifard SE, Bakouei A (2017) Self-templated synthesis of uniform nanoporous CuCo2O4 double-shelled hollow microspheres for high-performance asymmetric supercapacitors. Chem Commun 53:1052–1055
Zhao J, Li M, Sun J, Liu L, Su P, Yang Q, Li C (2012) Metal-oxide nanoparticles with desired morphology inherited from coordination-polymer precursors. Chemistry 18:3163–3168
Zhao J, Wang F, Su P, Li M, Chen J, Yang Q, Li C (2012) Spinel ZnMn2O4 nanoplate assemblies fabricated via “escape-by-crafty-scheme” strategy. J Mater Chem 22:13328–13333
Tao L, Huang M, Guo S, Wang Q, Li M, Xiao X, Cao G, Shao Y, Shen Y, Fu Y, Wang M (2019) Surface modification of NiCo2Te4 nanoclusters: a highly efficient electrocatalyst for overall water-splitting in neutral solution. Appl Catal B 254:424–431
Liu S, Hu L, Xu X, Al-Ghamdi AA, Fang X (2015) Nickel cobaltite nanostructures for photoelectric and catalytic applications. Small 11:4267–4283
Zheng W, Liu Y, Liu W, Ji H, Li F, Shen C, Fang X, Li X, Duan X (2021) A novel electrocatalytic filtration system with carbon nanotube supported nanoscale zerovalent copper toward ultrafast oxidation of organic pollutants. Water Res 194:116961
Chen H, Wang J, Han X, Liao F, Zhang Y, Gao L, Xu C (2019) Facile synthesis of mesoporous ZnCo2O4 hierarchical microspheres and their excellent supercapacitor performance. Ceram Int 45:8577–8584
Sun J, Du X, Wu R, Zhang Y, Xu C, Chen H (2020) Bundlelike CuCo2O4 microstructures assembled with ultrathin nanosheets as battery-type electrode materials for high-performance hybrid supercapacitors. ACS Appl Energy Mater 3:8026–8037
Lu Y, Dong CL, Huang YC, Zou Y, Liu Z, Liu Y, Li Y, He N, Shi J, Wang S (2020) Identifying the geometric site dependence of spinel oxides for the electrooxidation of 5-hydroxymethylfurfural. Angew Chem Int Ed Engl 59:19215–19221
Tao L, Guo P, Zhu W, Li T, Zhou X, Fu Y, Yu C, Ji H (2020) Highly efficient mixed-metal spinel cobaltite electrocatalysts for the oxygen evolution reaction. Chin J Catal 41:1855–1863
Han X, Sheng H, Yu C, Walker TW, Huber GW, Qiu J, Jin S (2020) Electrocatalytic oxidation of glycerol to formic acid by CuCo2O4 spinel oxide nanostructure catalysts. ACS Catal 10:6741–6752
Inamdar AI, Chavan HS, Hou B, Lee CH, Lee SU, Cha S, Kim H, Im H (2020) A robust nonprecious CuFe composite as a highly efficient bifunctional catalyst for overall electrochemical water splitting. Small 16:e1905884
Chavan HS, Lee CH, Inamdar AI, Han J, Park S, Cho S, Shreshta NK, Lee SU, Hou B, Im H, Kim H (2022) Designing and tuning the electronic structure of nickel-vanadium layered double hydroxides for highly efficient oxygen evolution electrocatalysis. ACS Catal 12:3821–3831
Acknowledgements
The authors gratefully acknowledge financial support from the National Basic Research Program of China (22078072 and 22272034), the Featured Innovation Project of Guangdong Education Department (2021ZDZX4060), Scientific Research Fund of Natural Science Foundation of Guangdong University of Petrochemical Technology (2019rc019, 2019rc011), Science and technology project of Maoming City (2020578, 2020526 and 2021018). Guangdong Basic and Applied Basic Research Foundation (2021A1515010305).
Author information
Authors and Affiliations
Contributions
LT designed the experiments. KP and LH helped to perform characterization and electrochemical measurements. HJ helped with the calculations by VASP. LT, MZ and CY analyzed the data and modified the manuscript. LT supervised the experiment.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical Approval
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Consent to Participate
All authors approved the version to be published.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tao, L., Pang, K., Huang, L. et al. Enhanced OER Performance by Cu Substituted Spinel Cobalt Oxide. Catal Lett 153, 2642–2650 (2023). https://doi.org/10.1007/s10562-022-04209-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-022-04209-7