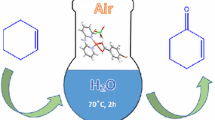
Abstract
Solvent effect plays a significant role in manipulating the chemical reactivity. As shown herein, selective oxidation of benzyl alcohol (BnOH) by 30 wt% hydrogen peroxide (H2O2) with iron(III) tosylate is a solvent-controlled reaction. The use of different solvents, dissimilar products can be obtained in the reaction: in chloroform, quantitative conversion to benzaldehyde (BnH) is achieved; while high yield is obtained when producing benzoic acid (BA) in acetonitrile. This phenomenon is related not only to the polarity of organic solvents, but also to the interaction energies between BnH and different solvents molecules. Molecular dynamics (MD) calculated by Materials Studio (Accelrys Software Inc., US) show a strong interaction between BnH and chloroform molecules, which means BnH (generated from the oxidation of BnOH) can be effectively protected by chloroform so as to prevent further oxidation to BA. Moreover, a free radical catalytic mechanism is verified in the oxidation of BnOH with H2O2 catalyzed by Fe(OTs)3·6H2O by a poisoning tests using BQ as the radical scavenger.
Graphical Abstract










Similar content being viewed by others
References
Larock RC (1999) Comprehensive organic transformations: a guide to functional group preparations. Wiley, New York
Dijksman A, Marino-González A, Payeras AMi, Arends IWCE, Sheldon RA (2001) J Am Chem Soc 123:6826–6833
Varma RS, Naicker KP (1999) Org Lett 1:189–192
Varma RS, Dahiya R (1997) Tetrahedron Lett 38:2043–2044
Namboodiri VV, Polshettiwar V, Varma RS (2007) Tetrahedron Lett 48:8839–8842
Azizi M, Maleki A, Hakimpoor F, Ghalavand R, Garavand A (2017) Catal Lett 147:2173–2177
Yu YY, Lu B, Wang XG, Zhao JX, Wang XZ, Cai QH (2010) Chem Eng J 162:738–742
Bothwell JM, Angeles VV, Carolan JP, Olson ME, Mohan RS (2010) Tetrahedron Lett 51:1056–1058
Olson ME, Carolan JP, Chiodo MV, Lazzara PR, Mohan RS (2010) Tetrahedron Lett 51:3969–3971
Baldwin NJ, Nord AN, O’Donnell BD, Mohan RS (2010) Tetrahedron Lett 53:6946–6949
Qian B, Zhang GY, Ding YZ, Huang HM (2013) Chem Commun 49:9839–9841
Spafford MJ, Anderson ED, Lacey JR, Palma AC, Mohan RS (2007) Tetrahedron Lett 48:8665–8667
Requies J, Güemez MB, Iriondo A, Barrio VL, Cambra JF, Arias PL (2012) Catal Lett 142:417–426
Cruz P, Pérez Y, Hierro ID, Fajardo M (2016) Micropor Mesopor Mat 220:136–147
Wu S, Ma HC, Lei ZQ (2016) Tetrahedron 66:8641–8647
Ni J, Yu WJ, He L, Sun H, Cao Y, He HY, Fan KN (2009) Green Chem 11:756–759
Cang RB, Lu B, Li XP, Niu R, Zhao JX, Cai QH (2015) Chem Eng Sci 137:268–275
Cánepa AL, Elías VR, Vaschetti VM, Sabre EV, Eimer GA, Casuscelli SG (2017) Appl Catal A-Gen 545:72–78
Xiong LS, Chen R, Chen FX (2016) RSC Adv 6:101048–101060
Hughes MD, Xu Y, Jenkins P, McMorn P, Landon P, Enache DI, Carley AF, Attard GA, Hutchings GJ, King F, Stitt EH, Johnston P, Griffin K, Kiely CJ (2005) Nature 437:1132–1135
Rautiainen S, Simakova O, Guo HF, Leino AR, Kordás K, Murzin D, Leskelä M, Repo T (2014) Appl Catal A-Gen 485:202–206
Yu C, Wu ST, Huang ZJ, Zhao Y, Zeng ZX, Xue WL (2016) J Mol Liq 224:139–145
Yu C, Huang ZJ, Zeng ZX, Xue WL (2016) J Solution Chem 45:395–409
Huang ZJ, Yu C, Xue WL, Lin FC, Zeng ZX (2017) Korean J Chem Eng 34:206–213
Yu C, Zeng ZX, Xue WL (2015) Ind Eng Chem Res 54:3961–3967
Zhu JJ, Figueiredo JL, Faria JL (2008) Catal Commun 9:2395–2397
Chaudhari MP, Sawant SB (2005) Chem Eng J 106:111–118
Zhao J, Yang JJ, Ma J (2014) Chem Eng J 239:171–177
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) J Phys Chem Ref Data 17:513–886
Bielski BHJ, Cabelli DE, Arudi RL, Ross AB (1985) J Phys Chem Ref Data 14:1041–1098
Kwan WP, Voelker BM (2002) Environ Sci Technol 36:1467–1476
Duesterberg CK, Waite TD (2006) Environ Sci Technol 40:4189–4195
Sha J, Zheng EJ, Zhou WJ, Liebens A, Pera-Titus M (2016) J Catal 337:199–207
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the contributing authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, Y., Yu, C., Wu, S. et al. Synthesis of Benzaldehyde and Benzoic Acid by Selective Oxidation of Benzyl Alcohol with Iron(III) Tosylate and Hydrogen Peroxide: A Solvent-Controlled Reaction. Catal Lett 148, 3082–3092 (2018). https://doi.org/10.1007/s10562-018-2515-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-018-2515-0