Abstract
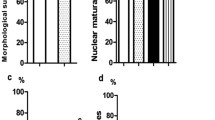
Oocyte banking is a vital step for safekeeping and spreading genetic resources of animals. It is also used for fertility preservation of human. Oocyte vitrification is closely related to the lower developmental competence which includes the cryo-injury arisen during vitrification. The aim of the present study was to evaluate the maturation, embryonic development and production of reactive oxygen species (ROS) of mice oocytes following the supplementation vitrification media with different concentrations of Ceratonia siliqua (carob) extracts. In this experimental study, germinal vesicle oocytes collected from 8 to 10 week-old female NMRI mice (30–40 gr) were randomly divided into six groups of vitrification media supplemented with 0 (control), 5, 10, 20, 30 and 50 µg/ml C. siliqua. After thawing, oocytes were put in an in vitro maturation medium (IVM) (α-MEM: Alpha Minimum Essential Medium). 3–4 and 24 h (hr) later, the oocyte nuclear maturity was checked. Standard in vitro fertilization was performed on the matured oocytes (MII), and embryonic development was followed. Extra- and intra-cellular ROS was measured in IVM medium after 24 h of oocyte incubation. The addition of 20 and 30 μg/ml C. siliqua extract to vitrification media improved normal morphology of warmed germinal vesicle (GV) oocytes, rate of germinal vesicle break down (GVBD), and metaphase 2 (MII) oocyte formation significantly (p < 0.05). Fertilization rate, (embryonic development to 2 cells stage, 4–8 cells stage, and > 8 cells stage increased in the 30 μg/ml C. siliqua group significantly (p < 0.05). Furthermore, supplementation of 30 μg/ml C. siliqua in vitrification media significantly decreased extra- and intra-cellular of ROS as well as embryonic fragmentation (p < 0.05). In conclusion, supplementation of GV oocyte vitrification media with carob extract improved maturation, fertilization, and embryonic development rate and decreased extra- and intra-cellular ROS levels.

Similar content being viewed by others
References
Al-Hasani S, Ozmen B et al (2007) Three years of routine vitrification of human zygotes: is it still fair to advocate slow-rate freezing? Reprod BioMed Online 14(3):288–293
Alzoubi KH, Alibbini S et al (2018) Carob (Ceratonia siliqua L.) prevents short-term memory deficit induced by chronic stress in rats. J Mol Neurosci 66(3):314–321
Amessis-Ouchemoukh N, Ouchemoukh S et al (2017) Bioactive metabolites involved in the antioxidant, anticancer and anticalpain activities of Ficus carica L., Ceratonia siliqua L. and Quercus ilex L. extracts. Ind Crops Prod 95:6–17
Borjizadeh A, Ahmadi H et al (2019) The effect of adding Rosmarinic and Ascorbic acids to vitrification media on fertilization rate of the mice oocyte: an experimental study. Int J Reprod BioMed 17(3):195–200
Brambillasca F, Guglielmo MC et al (2013) The current challenges to efficient immature oocyte cryopreservation. J Assist Reprod Genet 30(12):1531–1539
Cao Y, Xing Q et al (2009) Cryopreservation of immature and in vitro matured human oocytes by vitrification. Reprod Biomed Online 19(3):369–373
Channing CP, Pomerantz SH et al (1982) Actions of hormones and other factors upon oocyte maturation. Intraovarian control mechanisms. Springer, Berlin, pp 189–210
Comporti M (1989) Three models of free radical-induced cell injury. Chem Biol Interact 72(1–2):1–56
Corsi L, Avallone R et al (2002) Antiproliferative effects of Ceratonia siliqua L. on mouse hepatocellular carcinoma cell line. Fitoterapia 73(7–8):674–684
Curnow E, Ryan J et al (2010) In vitro developmental potential of macaque oocytes, derived from unstimulated ovaries, following maturation in the presence of glutathione ethyl ester. Hum Reprod 25(10):2465–2474
Custódio L, Escapa AL et al (2011) Phytochemical profile, antioxidant and cytotoxic activities of the carob tree (Ceratonia siliqua L.) germ flour extracts. Plant Foods Hum Nutr 66(1):78–84
Dinara S, Sengoku K et al (2001) Effects of supplementation with free radical scavengers on the survival and fertilization rates of mouse cryopreserved oocytes. Hum Reprod 16(9):1976–1981
Faramarzi A, Aghaz F et al (2019) Does supplementation of sperm freezing/thawing media with Ceratonia siliqua improve detrimental effect of cryopreservation on sperm parameters and chromatin quality in normozoospermic specimens? Cell Tissue Bank 20(3):403–409
Faramarzi A, Aghaz F et al (2020) In vitro application of Ceratonia siliqua improved sperm parameters and chromatin quality after vitrifacation in normozoospermic aged men. Middle East Fertil Soc J 24(1):6
Fasano G, Vannin AS et al (2010) Cryopreservation of human failed maturation oocytes shows that vitrification gives superior outcomes to slow cooling. Cryobiology 61(3):243–247
Gao C, Han HB et al (2012) Melatonin promotes embryonic development and reduces reactive oxygen species in vitrified mouse 2-cell embryos. J Pineal Res 52(3):305–311
Gardner DK, Sheehan CB et al (2007) Analysis of oocyte physiology to improve cryopreservation procedures. Theriogenology 67(1):64–72
Gupta MK, Uhm SJ et al (2010) Effect of vitrification and beta-mercaptoethanol on reactive oxygen species activity and in vitro development of oocytes vitrified before or after in vitro fertilization. Fertil Steril 93(8):2602–2607
Hadi MY, Hameed IH et al (2017) Ceratonia siliqua: characterization, pharmaceutical products and analysis of bioactive compounds: a review. Res J Pharm Technol 10(10):3585–3589
Hormonal R (2018) Fertility preservation: considerations for gender-diverse youth. Pubertal Suppr Transgender Youth 63:62–68
Huang J, Ma Y et al (2018) Dynamic changes in the global transcriptome of bovine germinal vesicle oocytes after vitrification followed by in vitro maturation. Reprod Fertil Dev 30(10):1298–1313
Izadi M, Vaghefi SHE et al (2018) Assessment of mouse oocytes ultrastructure following vitrification before and after in vitro maturation. Int J Morphol 36(1):430
Kang JT, Koo OJ et al (2009) Effects of melatonin on in vitro maturation of porcine oocyte and expression of melatonin receptor RNA in cumulus and granulosa cells. J Pineal Res 46(1):22–28
Kuwayama M, Vajta G et al (2005) Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online 11(3):300–308
Lane M, Maybach JM et al (2002) Addition of ascorbate during cryopreservation stimulates subsequent embryo development. Hum Reprod 17(10):2686–2693
Leibo S (2008) Cryopreservation of oocytes and embryos: optimization by theoretical versus empirical analysis. Theriogenology 69(1):37–47
Li Z, Gu R et al (2018) Preincubation with glutathione ethyl ester improves the developmental competence of vitrified mouse oocytes. J Assist Reprod Genet 35(7):1169–1178
Lucena E, Bernal DP et al (2006) Successful ongoing pregnancies after vitrification of oocytes. Fertil Steril 85(1):108–111
Ma Y, Pan B et al (2018) Expression of CD9 and CD81 in bovine germinal vesicle oocytes after vitrification followed by in vitro maturation. Cryobiology 81:206–209
Mandawala A, Harvey S et al (2016) Cryopreservation of animal oocytes and embryos: current progress and future prospects. Theriogenology 86(7):1637–1644
Matilla E, Martín-Cano FE et al (2019) N-acetylcysteine addition after vitrification improves oocyte mitochondrial polarization status and the quality of embryos derived from vitrified murine oocytes. BMC Vet Res 15(1):31
Men H, Monson RL et al (2003) Degeneration of cryopreserved bovine oocytes via apoptosis during subsequent culture. Cryobiology 47(1):73–81
Meziani S, Oomah BD et al (2015) Antibacterial activity of carob (Ceratonia siliqua L.) extracts against phytopathogenic bacteria Pectobacterium atrosepticum. Microb Pathog 78:95–102
Moawad AR, Fisher P et al (2012) In vitro fertilization of ovine oocytes vitrified by solid surface vitrification at germinal vesicle stage. Cryobiology 65(2):139–144
Moawad AR, Xu B et al (2014) L-carnitine supplementation during vitrification of mouse germinal vesicle stage–oocytes and their subsequent in vitro maturation improves meiotic spindle configuration and mitochondrial distribution in metaphase II oocytes. Hum Reprod 29(10):2256–2268
Noyes N, Labella PA et al (2010) Oocyte cryopreservation: a feasible fertility preservation option for reproductive age cancer survivors. J Assist Reprod Genet 27(8):495–499
Ott M, Gogvadze V et al (2007) Mitochondria, oxidative stress and cell death. Apoptosis 12(5):913–922
Pan B, Yang H et al (2018) Melatonin improves parthenogenetic development of vitrified–warmed mouse oocytes potentially by promoting G1/S cell cycle progression. Int J Mol Sci 19(12):4029
Paynter S (2000) Current status of the cryopreservation of human unfertilized oocytes. Hum Reprod Update 6(5):449–456
Rtibi K, Jabri MA et al (2015) Gastroprotective effect of carob (Ceratonia siliqua L.) against ethanol-induced oxidative stress in rat. BMC Complement Alternat Med 15(1):292
Sadeghzadeh F, Sadeghzadeh A et al (2020) The effect of hydro-alcoholic extract of Ceratonia Silique L. on spermatogenesis index in rats treated with cyclophosphamide: an experimental study. Int J Reprod BioMed 18(4):295
Saeedabadi S, Abazari-Kia AH et al (2018) Melatonin improves the developmental competence of goat oocytes. Int J Fertil Steril 12(2):157
Salzano A, Albero G et al (2014) Effect of resveratrol supplementation during culture on the quality and cryotolerance of bovine in vitro produced embryos. Anim Reprod Sci 151(3–4):91–96
Santos E, Appeltant R et al (2018) The effect of resveratrol on the developmental competence of porcine oocytes vitrified at germinal vesicle stage. Reprod Domest Anim 53(2):304–312
Shapiro BS, Daneshmand ST et al (2013) Matched-cohort comparison of single-embryo transfers in fresh and frozen-thawed embryo transfer cycles. Fertil Steril 99(2):389–392
Stoop D, Nekkebroeck J et al (2011) A survey on the intentions and attitudes towards oocyte cryopreservation for non-medical reasons among women of reproductive age. Hum Reprod 26(3):655–661
Vafaei A, Mohammadi S et al (2018) Effects of carob (Ceratonia siliqua) on sperm quality, testicular structure, testosterone level and oxidative stress in busulfan-induced infertile mice. Pharmaceut Sci 24(2):104–111
Wang Y, Zhang M et al (2018) Resveratrol promotes the embryonic development of vitrified mouse oocytes after in vitro fertilization. Vitro Cell Dev Biol Anim 54(6):430–438
Wu Z, Pan B et al (2019) Melatonin improves in vitro development of vitrified-warmed mouse germinal vesicle oocytes potentially via modulation of spindle assembly checkpoint-related genes. Cells 8(9):1009
Acknowledgements
The authors would like to thank the colleagues who helped with the experiments and data collection.
Funding
This study was funded by Kermanshah University of Medical Science (97365).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All applicable institutional guidelines for the care and use of animals were followed (IR.KUMS.REC.1397.292).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Faramarzi, A., Aghaz, F., Bakhtiari, M. et al. Ceratonia siliqua (Carob) extract improved in vitro development of vitrified-warmed mouse germinal vesicle oocytes: assessment of possible mechanism. Cell Tissue Bank 22, 137–144 (2021). https://doi.org/10.1007/s10561-020-09873-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-020-09873-w