Abstract
Purpose
Diabetic cardiomyopathy (DCM) is a common and severe complication of diabetes. Inflammation and oxidative stress play important roles in DCM development. Bicyclol is a hepatoprotective drug in China that exerts anti-inflammatory effects by inhibiting the MAPK and NF-κB pathways to prevent obesity-induced cardiomyopathy. Our purpose was to explore the effect and mechanism of bicyclol on DCM.
Methods
A type 1 diabetes mouse model was established using C57BL/6 mice by intraperitoneal injection of STZ. The therapeutic effect of bicyclol was evaluated in both heart tissues of diabetic mice and high concentration of glucose (HG)-stimulated H9c2 cells.
Results
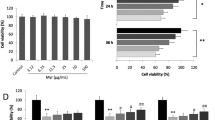
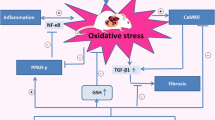
We showed that bicyclol significantly attenuated diabetes-induced cardiac hypertrophy and fibrosis, which is accompanied by the preservation of cardiac function in mice. In addition, bicyclol exhibited anti-inflammatory and anti-oxidative effects both in vitro and in vivo. Furthermore, bicyclol inhibited the hyperglycemia-induced activation of MAPKs and NF-κB pathways, while upregulating the Nrf-2/HO-1 pathway to exhibit protective effects.
Conclusion
Our data indicate that bicyclol could be a promising cardioprotective agent in the treatment of DCM.







Similar content being viewed by others
Data Availability
All the data in this study are available upon reasonable request from the corresponding author.
Code Availability
Not applicable.
Abbreviations
- BIC:
-
bicyclol
- BNP:
-
brain natriuretic peptide
- BSA:
-
bovine serum albumin
- CK-MB:
-
creatine kinase-MB
- CMC-Na:
-
Carboxymethylcellulose sodium
- Col-1:
-
type 1 collagen
- DAB:
-
diaminobenzidine
- DCM:
-
diabetic cardiomyopathy
- DM:
-
diabetes mellitus
- DMSO:
-
dimethylsulphoxide
- DMEM:
-
Dulbecco’s Modified Eagle’s Medium
- EF:
-
ejection fraction
- ERK:
-
extracellular regulated protein kinases
- FBS:
-
fetal bovine serum
- FS:
-
fractional shortening
- HG:
-
high concentration glucose
- HO-1:
-
heme oxygenase-1
- IL-1β:
-
interleukin 1β
- IL-6:
-
interleukin 6
- IκB-α:
-
inhibitor of NF-κB-α
- JNK:
-
c-Jun N-terminal kinase
- Keap-1:
-
Kelch-like ECH-associated protein 1
- LDH:
-
lactic dehydrogenase
- MAPK:
-
mitogen-activated protein kinase
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazoliumbromide
- NAFLD:
-
nonalcoholic fatty liver
- NF-κB:
-
nuclear factor kappa-B
- Nrf-2:
-
nuclear factor-erythroid 2-related factor 2
- ROS:
-
reactive oxygen species
- STZ:
-
streptozotocin
- T1DM:
-
type 1 diabetes mellitus
- TGF-β:
-
transforming growth factor-β
- TNF-α:
-
tumor necrosis factor-α
- β-Myhc:
-
β-myosin heavy chain
References
Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81.
de Ferranti SD, de Boer IH, Fonseca V, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2014;37(10):2843–63.
Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 2016;12(3):144–53.
Ritchie RH, Abel ED. Basic Mechanisms of Diabetic Heart Disease. Circ Res. 2020;126(11):1501–25.
Marwick TH, Ritchie R, Shaw JE, Kaye D. Implications of underlying mechanisms for the recognition and management of diabetic cardiomyopathy. J Am Coll Cardiol. 2018;71(3):339–51.
Palomer X, Salvadó L, Barroso E, Vázquez-Carrera M. An overview of the crosstalk between inflammatory processes and metabolic dysregulation during diabetic cardiomyopathy. Int J Cardiol. 2013;168(4):3160–72.
Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57(4):660–71.
Bou-Teen D, Kaludercic N, Weissman D, et al. Mitochondrial ROS and mitochondria-targeted antioxidants in the aged heart. Free Radic Biol Med. 2021;167:109–24.
Rendra E, Riabov V, Mossel DM, et al. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology. 2019;224(2):242–53.
Wang YH, Qiu C, Wang DW, et al. Identification of multiple constituents in the traditional Chinese medicine formula Sheng-Mai San and rat plasma after oral administration by HPLC-DAD-MS/MS. J Pharm Biomed Anal. 2011;54(5):1110–27.
Tian J, Tang W, Xu M, et al. Shengmai San alleviates diabetic Ccardiomyopathy through improvement of mitochondrial lipid metabolic disorder. Cell Physiol Biochem. 2018;50(5):1726–39.
Zhao T, Mao L, Yu Z, et al. Therapeutic potential of bicyclol in liver diseases: lessons from a synthetic drug based on herbal derivative in traditional Chinese medicine. Int Immunopharmacol. 2021;91:107308.
Yao XM, Li Y, Li HW, et al. Bicyclol attenuates tetracycline-induced fatty liver associated with inhibition of hepatic ER stress and apoptosis in mice. Can J Physiol Pharmacol. 2016;94(1):1–8.
Cui J, Li Z, Qian LB, et al. Reducing the oxidative stress mediates the cardioprotection of bicyclol against ischemia-reperfusion injury in rats. J Zhejiang Univ Sci B. 2013;14(6):487–95.
Zhao W, Yan Y, Xiao Z, et al. Bicyclol ameliorates nonalcoholic fatty liver disease in mice via inhibiting MAPKs and NF-kappaB signaling pathways. Biomed Pharmacother. 2021;141:111874.
Zhang Y, Liu Z, Wu J, et al. New MD2 inhibitors derived from curcumin with improved anti-inflammatory activity. Eur J Med Chem. 2018;148:291–305.
Chen Y, Lin W, Zhong L, et al. Bicyclol attenuates obesity-induced cardiomyopathy via inhibiting NF-kappaB and MAPK signaling pathways. Cardiovasc Drugs Ther. 2022. online ahead of print.
You S, Qian J, Sun C, et al. An Aza resveratrol-chalcone derivative 6b protects mice against diabetic cardiomyopathy by alleviating inflammation and oxidative stress. J Cell Mol Med. 2018;22(3):1931–43.
Li L, Luo W, Qian Y, et al. Luteolin protects against diabetic cardiomyopathy by inhibiting NF-kappaB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine. 2019;59:152774.
Gao Y, Liu W, Su X, et al. The beneficial effects of Chinese herbal monomers on ameliorating diabetic cardiomyopathy via Nrf2 signaling. Oxidative Med Cell Longev. 2022;2022:3959390.
Tian J, Zhao Y, Liu Y, et al. Roles and mechanisms of herbal medicine for diabetic cardiomyopathy: current status and perspective. Oxidative Med Cell Longev. 2017;2017:8214541.
Liu X, Guo B, Zhang W, Ma B, Li Y. MiR-20a-5p overexpression prevented diabetic cardiomyopathy via inhibition of cardiomyocyte apoptosis, hypertrophy, fibrosis and JNK/NF-kappaB signalling pathway. J Biochem. 2021;170(3):349–62.
Ge Q, Zhao L, Ren XM, Ye P, Hu ZY. LCZ696, an angiotensin receptor-neprilysin inhibitor, ameliorates diabetic cardiomyopathy by inhibiting inflammation, oxidative stress and apoptosis. Exp Biol Med (Maywood). 2019;244(12):1028–39.
Gao Y, Kang L, Li C, et al. Resveratrol ameliorates diabetes-induced cardiac dysfunction through AT1R-ERK/p38 MAPK signaling pathway. Cardiovasc Toxicol. 2016;16(2):130–7.
Zuo G, Ren X, Qian X, et al. Inhibition of JNK and p38 MAPK-mediated inflammation and apoptosis by ivabradine improves cardiac function in streptozotocin-induced diabetic cardiomyopathy. J Cell Physiol. 2019;234(2):1925–36.
Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia. 2018;61(1):21–8.
Ceriello A. Oxidative stress and diabetes-associated complications. Endocr Pract. 2006;12(Suppl 1):60–2.
Anderson EJ, Kypson AP, Rodriguez E, et al. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J Am Coll Cardiol. 2009;54(20):1891–8.
Saha S, Buttari B, Panieri E, Profumo E, Saso L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules. 2020;25(22):5474.
Wu X, Huang L, Liu J. Relationship between oxidative stress and nuclear factor-erythroid-2-related factor 2 signaling in diabetic cardiomyopathy. Exp Ther Med. 2021;22(1):678.
Balligand JL. Reducing damage through Nrf-2. Cardiovasc Res. 2013;100(1):1–3.
Gupta A, Behl T, Sehgal A, et al. Therapeutic potential of Nrf-2 pathway in the treatment of diabetic neuropathy and nephropathy. Mol Biol Rep. 2021;48(3):2761–74.
Luo J, Yan D, Li S, et al. Allopurinol reduces oxidative stress and activates Nrf2/p62 to attenuate diabetic cardiomyopathy in rats. J Cell Mol Med. 2020;24(2):1760–73.
Elmarakby AA, Sullivan JC. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc Ther. 2012;30(1):49–59.
Zhang Y, Ren J. Epigenetics and obesity cardiomyopathy: From pathophysiology to prevention and management. Pharmacol Ther. 2016;161:52–66.
Zhang M, Lin J, Wang S, et al. Melatonin protects against diabetic cardiomyopathy through Mst1/Sirt3 signaling. J Pineal Res. 2017;63(2):e12418.
Bao XQ, Liu GT. Bicyclol protects HepG2 cells against D-galactosamine-induced apoptosis through inducing heat shock protein 27 and mitochondria associated pathway. Acta Pharmacol Sin. 2010;31(2):219–26.
Jia S, Jin L, Cheng X, et al. Bicyclol alleviates high-fat diet-induced hepatic ER stress- and autophagy-associated non-alcoholic fatty liver disease/non-alcoholic steatohepatitis in mice. Drug Dev Ind Pharm. 2022;48(6):247–54.
Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98.
Henstridge DC, Bruce CR, Pang CP, et al. Skeletal muscle-specific overproduction of constitutively activated c-Jun N-terminal kinase (JNK) induces insulin resistance in mice. Diabetologia. 2012;55(10):2769–78.
Li H, Xu Q, Xu C, et al. Bicyclol regulates hepatic gluconeogenesis in rats with type 2 diabetes and non-alcoholic fatty liver disease by inhibiting inflammation. Front Pharmacol. 2021;12:644129.
Funding
This study was supported by the National Natural Science Foundation of China (82170373 to Y.W.).
Author information
Authors and Affiliations
Contributions
Yi Wang and Ying He contributed to the literature search and study design. Lingxi Zhang, Chenghong Hu, Bo Jin, Jing Liao, Bin Bai, Leiming Jin, Minxiu Wang, Weiwei Zhu, Xuelian Xu and Li Zheng carried out experiments. Lingxi Zhang, Xuedan Wu and Yongsheng Jiang contributed to data collection and analysis. Lingxi Zhang participated in the drafting of the article. Yi Wang and Ying He revised the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Statement
All animal experiments were in compliance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Welfare Committee of Wenzhou Medical University (Approved ID: wydw2018-0124).
Consent for Publication
Not applicable.
Consent to Participate
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Mote
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 76 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, L., Hu, C., Jin, B. et al. Bicyclol Alleviates Streptozotocin-induced Diabetic Cardiomyopathy By Inhibiting Chronic Inflammation And Oxidative Stress. Cardiovasc Drugs Ther 38, 555–568 (2024). https://doi.org/10.1007/s10557-023-07426-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-023-07426-3