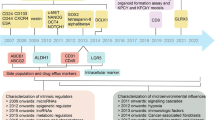
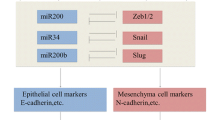
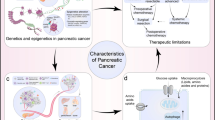
Abstract
Cellular plasticity and therapy resistance are critical features of pancreatic cancer, a highly aggressive and fatal disease. The pancreas, a vital organ that produces digestive enzymes and hormones, is often affected by two main types of cancer: the pre-dominant ductal adenocarcinoma and the less common neuroendocrine tumors. These cancers are difficult to treat due to their complex biology characterized by cellular plasticity leading to therapy resistance. Cellular plasticity refers to the capability of cancer cells to change and adapt to different microenvironments within the body which includes acinar-ductal metaplasia, epithelial to mesenchymal/epigenetic/metabolic plasticity, as well as stemness. This plasticity allows heterogeneity of cancer cells, metastasis, and evasion of host’s immune system and develops resistance to radiation, chemotherapy, and targeted therapy. To overcome this resistance, extensive research is ongoing exploring the intrinsic and extrinsic factors through cellular reprogramming, chemosensitization, targeting metabolic, key survival pathways, etc. In this review, we discussed the mechanisms of cellular plasticity involving cellular adaptation and tumor microenvironment and provided a comprehensive understanding of its role in therapy resistance and ways to overcome it.




Similar content being viewed by others
References
Mizrahi, J. D., et al. (2020). Pancreatic cancer. Lancet, 395(10242), 2008–2020.
Siegel, R. L., et al. (2023). Cancer statistics, 2023. CA: A Cancer Journal for Clinicians, 73(1), 17–48.
Hidalgo, M., et al. (2015). Addressing the challenges of pancreatic cancer: Future directions for improving outcomes. Pancreatology, 15(1), 8–18.
Schober, M., et al. (2014). Desmoplasia and chemoresistance in pancreatic cancer. Cancers (Basel), 6(4), 2137–2154.
WHO Classification of Tumours Editorial Board. (2019). Digestive System Tumours: WHO Classification of Tumours, 5th ed. Vol. 1.
Stewart, B. W., et al. (2014). World Cancer Report 2014: World Cancer Reports.
Pishvaian, M. J., & Brody, J. R. (2017). Therapeutic implications of molecular subtyping for pancreatic cancer. Oncology (Williston Park), 31(3), 159–66. 168.
Fitzgerald, T. L., et al. (2008). Changing incidence of pancreatic neoplasms: A 16-year review of statewide tumor registry. Pancreas, 37(2), 134–138.
Siegel, R. L., et al. (2022). Cancer statistics, 2022. CA: A Cancer Journal for Clinicians, 72(1), 7–33.
Gittes, G. K. (2009). Developmental biology of the pancreas: A comprehensive review. Developmental Biology, 326(1), 4–35.
Rhim, A. D., et al. (2012). EMT and dissemination precede pancreatic tumor formation. Cell, 148(1–2), 349–361.
Farrell, A. S., et al. (2017). MYC regulates ductal-neuroendocrine lineage plasticity in pancreatic ductal adenocarcinoma associated with poor outcome and chemoresistance. Nature Communications, 8(1), 1728.
Reichert, M., et al. (2018). Regulation of epithelial plasticity determines metastatic organotropism in pancreatic cancer. Developmental Cell, 45(6), 696-711 e8.
Crawford, H. C., Pasca di Magliano, M., & Banerjee, S. (2019). Signaling networks that control cellular plasticity in pancreatic tumorigenesis, progression, and metastasis. Gastroenterology., 156(7), 2073–2084.
Shen, S., & Clairambault, J. (2020). Cell plasticity in cancer cell populations. F1000Res, 9, 635.
Yuan, S., Norgard, R. J., & Stanger, B. Z. (2019). Cellular plasticity in cancer. Cancer Discovery, 9(7), 837–851.
Rambow, F., Marine, J. C., & Goding, C. R. (2019). Melanoma plasticity and phenotypic diversity: Therapeutic barriers and opportunities. Genes & Development, 33(19–20), 1295–1318.
Qin, S., et al. (2020). Emerging role of tumor cell plasticity in modifying therapeutic response. Signal Transduction and Targeted Therapy, 5(1), 228.
Kemper, K., et al. (2014). Phenotype switching: Tumor cell plasticity as a resistance mechanism and target for therapy. Cancer Research, 74(21), 5937–5941.
Gupta, P. B., et al. (2019). Phenotypic plasticity: Driver of cancer initiation, progression, and therapy resistance. Cell Stem Cell, 24(1), 65–78.
Zhuang, X., Zhang, H., & Hu, G. (2019). Cancer and microenvironment plasticity: Double-edged swords in metastasis. Trends in Pharmacological Sciences, 40(6), 419–429.
Smigiel, J. M., et al. (2019). Cellular plasticity and metastasis in breast cancer: A pre- and post-malignant problem. Journal of Cancer Metastasis and Treatment, 5, 47.
Contreras-Trujillo, H., et al. (2021). Deciphering intratumoral heterogeneity using integrated clonal tracking and single-cell transcriptome analyses. Nature Communications, 12(1), 6522.
Li, M., et al. (2020). An algorithm to quantify intratumor heterogeneity based on alterations of gene expression profiles. Communications Biology, 3(1), 505.
Hinohara, K., & Polyak, K. (2019). Intratumoral heterogeneity: More than just mutations. Trends in Cell Biology, 29(7), 569–579.
Sun, X. X., & Yu, Q. (2015). Intra-tumor heterogeneity of cancer cells and its implications for cancer treatment. Acta Pharmacologica Sinica, 36(10), 1219–1227.
Nabi, K., & Le, A. (2021). The intratumoral heterogeneity of cancer metabolism. Advances in Experimental Medicine and Biology, 1311, 149–160.
Xiao, Z., Dai, Z., & Locasale, J. W. (2019). Metabolic landscape of the tumor microenvironment at single cell resolution. Nature Communications, 10(1), 3763.
Lawson, D. A., et al. (2018). Tumour heterogeneity and metastasis at single-cell resolution. Nature Cell Biology, 20(12), 1349–1360.
da Silva-Diz, V., et al. (2018). Cancer cell plasticity: Impact on tumor progression and therapy response. Seminars in Cancer Biology, 53, 48–58.
Thiery, J. P. (2002). Epithelial-mesenchymal transitions in tumour progression. Nature Reviews Cancer, 2(6), 442–454.
Polyak, K., & Weinberg, R. A. (2009). Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nature Reviews Cancer, 9(4), 265–273.
Farmer, P., et al. (2009). A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nature Medicine, 15(1), 68–74.
Shibue, T., & Weinberg, R. A. (2017). EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nature Reviews. Clinical Oncology, 14(10), 611–629.
Byers, L. A., et al. (2013). An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clinical Cancer Research, 19(1), 279–290.
Horn, L. A., Fousek, K., & Palena, C. (2020). Tumor plasticity and resistance to immunotherapy. Trends in Cancer, 6(5), 432–441.
Baccelli, I., et al. (2013). Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nature Biotechnology, 31(6), 539–544.
Aktas, B., et al. (2009). Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Research, 11(4), R46.
Micalizzi, D. S., et al. (2009). The Six1 homeoprotein induces human mammary carcinoma cells to undergo epithelial-mesenchymal transition and metastasis in mice through increasing TGF-beta signaling. The Journal of Clinical Investigation, 119(9), 2678–2690.
Kong, D., Hughes, C. J., & Ford, H. L. (2020). Cellular plasticity in breast cancer progression and therapy. Frontiers in Molecular Biosciences, 7, 72.
Ayob, A. Z., & Ramasamy, T. S. (2018). Cancer stem cells as key drivers of tumour progression. Journal of Biomedical Science, 25(1), 20.
PerusinaLanfranca, M., et al. (2020). Interleukin 22 signaling regulates acinar cell plasticity to promote pancreatic tumor development in mice. Gastroenterology, 158(5), 1417-1432 e11.
Quilichini, E., et al. (2019). Pancreatic ductal deletion of Hnf1b disrupts exocrine homeostasis, leads to pancreatitis, and facilitates tumorigenesis. Cellular and Molecular Gastroenterology and Hepatology, 8(3), 487–511.
Tanaka, M., et al. (2012). International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology, 12(3), 183–197.
Strobel, O., et al. (2007). Beta cell transdifferentiation does not contribute to preneoplastic/metaplastic ductal lesions of the pancreas by genetic lineage tracing in vivo. Proceedings of the National Academy of Sciences of the United States of America, 104(11), 4419–4424.
Grippo, P. J., et al. (2003). Preinvasive pancreatic neoplasia of ductal phenotype induced by acinar cell targeting of mutant Kras in transgenic mice. Cancer Research, 63(9), 2016–2019.
Tuveson, D. A., et al. (2006). Mist1-KrasG12D knock-in mice develop mixed differentiation metastatic exocrine pancreatic carcinoma and hepatocellular carcinoma. Cancer Research, 66(1), 242–247.
Liou, G. Y., et al. (2013). Macrophage-secreted cytokines drive pancreatic acinar-to-ductal metaplasia through NF-kappaB and MMPs. Journal of Cell Biology, 202(3), 563–577.
Logsdon, C. D., & Ji, B. (2009). Ras activity in acinar cells links chronic pancreatitis and pancreatic cancer. Clinical Gastroenterology and Hepatology, 7(11 Suppl), S40–S43.
Liou, G. Y., et al. (2016). Mutant KRas-induced mitochondrial oxidative stress in acinar cells upregulates EGFR signaling to drive formation of pancreatic precancerous lesions. Cell Reports, 14(10), 2325–2336.
Hezel, A. F., et al. (2008). Pancreatic LKB1 deletion leads to acinar polarity defects and cystic neoplasms. Molecular and Cellular Biology, 28(7), 2414–2425.
Sandgren, E. P., et al. (1990). Overexpression of TGF alpha in transgenic mice: Induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell, 61(6), 1121–1135.
Liu, J., et al. (2016). TGF-beta1 promotes acinar to ductal metaplasia of human pancreatic acinar cells. Science and Reports, 6, 30904.
Liou, G. Y., et al. (2015). Protein kinase D1 drives pancreatic acinar cell reprogramming and progression to intraepithelial neoplasia. Nature Communications, 6, 6200.
Means, A. L., et al. (2005). Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development, 132(16), 3767–3776.
Shi, G., et al. (2013). Maintenance of acinar cell organization is critical to preventing Kras-induced acinar-ductal metaplasia. Oncogene, 32(15), 1950–1958.
Wei, D., et al. (2016). KLF4 is essential for induction of cellular identity change and acinar-to-ductal reprogramming during early pancreatic carcinogenesis. Cancer Cell, 29(3), 324–338.
Baer, R., et al. (2014). Pancreatic cell plasticity and cancer initiation induced by oncogenic Kras is completely dependent on wild-type PI 3-kinase p110alpha. Genes & Development, 28(23), 2621–2635.
Wu, C. Y., et al. (2014). PI3K regulation of RAC1 is required for KRAS-induced pancreatic tumorigenesis in mice. Gastroenterology, 147(6), 1405–16 e7.
Payne, S. N., et al. (2015). PIK3CA mutations can initiate pancreatic tumorigenesis and are targetable with PI3K inhibitors. Oncogenesis, 4(10), e169.
Hill, R., et al. (2010). PTEN loss accelerates KrasG12D-induced pancreatic cancer development. Cancer Research, 70(18), 7114–7124.
Kopp, J. L., et al. (2012). Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell, 22(6), 737–750.
Ardito, C. M., et al. (2012). EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell, 22(3), 304–317.
Ji, B., et al. (2009). Ras activity levels control the development of pancreatic diseases. Gastroenterology, 137(3), 1072–82. 1082 e1-6.
Navas, C., et al. (2012). EGF receptor signaling is essential for k-ras oncogene-driven pancreatic ductal adenocarcinoma. Cancer Cell, 22(3), 318–330.
Guerra, C., et al. (2011). Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell, 19(6), 728–739.
Guerra, C., et al. (2007). Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell, 11(3), 291–302.
Liou, G. Y., et al. (2015). Mutant KRAS-induced expression of ICAM-1 in pancreatic acinar cells causes attraction of macrophages to expedite the formation of precancerous lesions. Cancer Discovery, 5(1), 52–63.
Krebs, A. M., et al. (2017). The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nature Cell Biology, 19(5), 518–529.
Cruz, V. H., et al. (2019). Axl-mediated activation of TBK1 drives epithelial plasticity in pancreatic cancer. JCI Insight, 5(9), e126117.
Aguilera, K. Y., et al. (2014). Collagen signaling enhances tumor progression after anti-VEGF therapy in a murine model of pancreatic ductal adenocarcinoma. Cancer Research, 74(4), 1032–1044.
Shintani, Y., et al. (2008). Collagen I-mediated up-regulation of N-cadherin requires cooperative signals from integrins and discoidin domain receptor 1. Journal of Cell Biology, 180(6), 1277–1289.
Suzuki, K., et al. (2017). Metadherin promotes metastasis by supporting putative cancer stem cell properties and epithelial plasticity in pancreatic cancer. Oncotarget, 8(39), 66098–66111.
Jeon, H. Y., et al. (2010). Expression patterns of astrocyte elevated gene-1 (AEG-1) during development of the mouse embryo. Gene Expression Patterns, 10(7–8), 361–367.
Venugopal, A., et al. (2022). EMT molecular signatures of pancreatic neuroendocrine neoplasms. International Journal of Molecular Sciences, 23(21), 13645.
Zhou, B., et al. (2021). High vimentin expression with E-cadherin expression loss predicts a poor prognosis after resection of grade 1 and 2 pancreatic neuroendocrine tumors. BMC Cancer, 21(1), 334.
Ikezono, Y., et al. (2017). Pancreatic neuroendocrine tumors and EMT behavior are driven by the CSC marker DCLK1. Molecular Cancer Research, 15(6), 744–752.
Adamska, A., & Falasca, M. (2018). Epithelial plasticity is crucial for pancreatic cancer metastatic organotropism. Annals of Translational Medicine, 6(Suppl 1), S53.
Aiello, N. M., et al. (2018). EMT subtype influences epithelial plasticity and mode of cell migration. Dev Cell, 45(6), 681-695 e4.
Storz, P. (2017). Acinar cell plasticity and development of pancreatic ductal adenocarcinoma. Nature Reviews. Gastroenterology & Hepatology, 14(5), 296–304.
Gidekel Friedlander, S. Y., et al. (2009). Context-dependent transformation of adult pancreatic cells by oncogenic K-Ras. Cancer Cell, 16(5), 379–389.
Morris, JPt., et al. (2010). Beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. Journal of Clinical Investigation, 120(2), 508–20.
Alonso-Curbelo, D., et al. (2021). A gene-environment-induced epigenetic program initiates tumorigenesis. Nature, 590(7847), 642–648.
Del Poggetto, E., et al. (2021). Epithelial memory of inflammation limits tissue damage while promoting pancreatic tumorigenesis. Science, 373(6561), eabj0486.
Li, Y., et al. (2021). Mutant Kras co-opts a proto-oncogenic enhancer network in inflammation-induced metaplastic progenitor cells to initiate pancreatic cancer. Nature Cancer, 2(1), 49–65.
Burdziak, C., et al. (2023). Epigenetic plasticity cooperates with cell-cell interactions to direct pancreatic tumorigenesis. Science, 380(6645), eadd5327.
Flavahan, W. A., Gaskell, E., Bernstein, B. E. (2017). Epigenetic plasticity and the hallmarks of cancer. Science, 357(6348), eaal2380.
Dawson, M. A. (2017). The cancer epigenome: Concepts, challenges, and therapeutic opportunities. Science, 355(6330), 1147–1152.
Xie, W., et al. (2013). Epigenomic analysis of multilineage differentiation of human embryonic stem cells. Cell, 153(5), 1134–1148.
Gifford, C. A., et al. (2013). Transcriptional and epigenetic dynamics during specification of human embryonic stem cells. Cell, 153(5), 1149–1163.
Vander Heiden, M. G., Cantley, L. C., & Thompson, C. B. (2009). Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science., 324(5930), 1029–33.
Olivares, O., et al. (2017). Collagen-derived proline promotes pancreatic ductal adenocarcinoma cell survival under nutrient limited conditions. Nature Communications, 8, 16031.
Guillaumond, F., et al. (2013). Strengthened glycolysis under hypoxia supports tumor symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma. Proceedings of the National Academy of Sciences of the United States of America, 110(10), 3919–3924.
Lyssiotis, C. A., & Kimmelman, A. C. (2017). Metabolic interactions in the tumor microenvironment. Trends in Cell Biology, 27(11), 863–875.
Sousa, C. M., & Kimmelman, A. C. (2014). The complex landscape of pancreatic cancer metabolism. Carcinogenesis, 35(7), 1441–1450.
Perera, R. M., & Bardeesy, N. (2015). Pancreatic cancer metabolism: Breaking it down to build it back up. Cancer Discovery, 5(12), 1247–1261.
Blum, R., & Kloog, Y. (2014). Metabolism addiction in pancreatic cancer. Cell Death & Disease, 5(2), e1065.
Liang, C., et al. (2016). Metabolic plasticity in heterogeneous pancreatic ductal adenocarcinoma. Biochimica et Biophysica Acta, 1866(2), 177–188.
Bryant, K. L., et al. (2014). KRAS: Feeding pancreatic cancer proliferation. Trends in Biochemical Sciences, 39(2), 91–100.
Ying, H., et al. (2012). Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell, 149(3), 656–670.
Slawson, C., Copeland, R. J., & Hart, G. W. (2010). O-GlcNAc signaling: A metabolic link between diabetes and cancer? Trends in Biochemical Sciences, 35(10), 547–555.
Stincone, A., et al. (2015). The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biological Reviews of the Cambridge Philosophical Society, 90(3), 927–963.
Neesse, A., et al. (2011). Stromal biology and therapy in pancreatic cancer. Gut, 60(6), 861–868.
Casazza, A., et al. (2014). Tumor stroma: A complexity dictated by the hypoxic tumor microenvironment. Oncogene, 33(14), 1743–1754.
Kalluri, R., & Zeisberg, M. (2006). Fibroblasts in cancer. Nature Reviews Cancer, 6(5), 392–401.
Xing, Y., et al. (2015). Metabolic reprogramming of the tumour microenvironment. FEBS Journal, 282(20), 3892–3898.
Yoshida, G. J. (2015). Metabolic reprogramming: The emerging concept and associated therapeutic strategies. Journal of Experimental & Clinical Cancer Research, 34, 111.
Zhao, H., et al. (2016). Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. eLife, 5, e10250.
Jaster, R. (2004). Molecular regulation of pancreatic stellate cell function. Molecular Cancer, 3, 26.
Sada, M., et al. (2016). Hypoxic stellate cells of pancreatic cancer stroma regulate extracellular matrix fiber organization and cancer cell motility. Cancer Letters, 372(2), 210–218.
Lisanti, M. P., Martinez-Outschoorn, U. E., & Sotgia, F. (2013). Oncogenes induce the cancer-associated fibroblast phenotype: Metabolic symbiosis and “fibroblast addiction” are new therapeutic targets for drug discovery. Cell Cycle, 12(17), 2723–2732.
Aponte, P. M., & Caicedo, A. (2017). Stemness in cancer: Stem cells, cancer stem cells, and their microenvironment. Stem Cells International, 2017, 5619472.
Li, C., et al. (2007). Identification of pancreatic cancer stem cells. Cancer Research, 67(3), 1030–1037.
Patil, K., et al. (2021). The plasticity of pancreatic cancer stem cells: Implications in therapeutic resistance. Cancer and Metastasis Reviews, 40(3), 691–720.
Di Carlo, C., Brandi, J., & Cecconi, D. (2018). Pancreatic cancer stem cells: Perspectives on potential therapeutic approaches of pancreatic ductal adenocarcinoma. World Journal of Stem Cells, 10(11), 172–182.
Hermann, P. C., et al. (2007). Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell, 1(3), 313–323.
Nair, N., et al. (2017). A cancer stem cell model as the point of origin of cancer-associated fibroblasts in tumor microenvironment. Science and Reports, 7(1), 6838.
Calle, A. S., et al. (2016). A new PDAC mouse model originated from iPSCs-converted pancreatic cancer stem cells (CSCcm). American Journal of Cancer Research, 6(12), 2799–2815.
Hassan, G., et al. (2022). Different pancreatic cancer microenvironments convert iPSCs into cancer stem cells exhibiting distinct plasticity with altered gene expression of metabolic pathways. Journal of Experimental & Clinical Cancer Research, 41(1), 29.
Gaur, P., et al. (2011). Identification of cancer stem cells in human gastrointestinal carcinoid and neuroendocrine tumors. Gastroenterology, 141(5), 1728–1737.
Krampitz, G. W., et al. (2016). Identification of tumorigenic cells and therapeutic targets in pancreatic neuroendocrine tumors. Proceedings of the National Academy of Sciences of the United States of America, 113(16), 4464–4469.
Katsuta, E., et al. (2016). CD73 as a therapeutic target for pancreatic neuroendocrine tumor stem cells. International Journal of Oncology, 48(2), 657–669.
Truong, L. H., & Pauklin, S. (2021). Pancreatic cancer microenvironment and cellular composition: Current understandings and therapeutic approaches. Cancers (Basel), 13(19), 5028.
Ramon, Y. C. S., et al. (2020). Clinical implications of intratumor heterogeneity: Challenges and opportunities. Journal of Molecular Medicine (Berlin, Germany), 98(2), 161–177.
Lecharpentier, A., et al. (2011). Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. British Journal of Cancer, 105(9), 1338–1341.
Dongre, A., & Weinberg, R. A. (2019). New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nature Reviews Molecular Cell Biology, 20(2), 69–84.
Fendt, S. M., Frezza, C., & Erez, A. (2020). Targeting metabolic plasticity and flexibility dynamics for cancer therapy. Cancer Discovery, 10(12), 1797–1807.
Venkatesan, S., et al. (2017). Treatment-induced mutagenesis and selective pressures sculpt cancer evolution. Cold Spring Harbor Perspectives in Medicine, 7(8), a026617.
Porter, R. L., et al. (2019). Epithelial to mesenchymal plasticity and differential response to therapies in pancreatic ductal adenocarcinoma. Proceedings of the National Academy of Sciences of the United States of America, 116(52), 26835–26845.
Bailey, P., et al. (2016). Genomic analyses identify molecular subtypes of pancreatic cancer. Nature, 531(7592), 47–52.
Collisson, E. A., et al. (2011). Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nature Medicine, 17(4), 500–503.
Moffitt, R. A., et al. (2015). Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nature Genetics, 47(10), 1168–1178.
Kloesch, B., et al. (2022). A GATA6-centred gene regulatory network involving HNFs and DeltaNp63 controls plasticity and immune escape in pancreatic cancer. Gut, 71(4), 766–777.
Thankamony, A. P., et al. (2020). Cancer stem cell plasticity - a deadly deal. Frontiers in Molecular Biosciences, 7, 79.
Castelli, V., et al. (2021). The great escape: The power of cancer stem cells to evade programmed cell death. Cancers (Basel), 13(2), 328.
Ciardiello, C., Leone, A., & Budillon, A. (2018). The crosstalk between cancer stem cells and microenvironment is critical for solid tumor progression: The significant contribution of extracellular vesicles. Stem Cells International, 2018, 6392198.
Ye, J., et al. (2014). The cancer stem cell niche: Cross talk between cancer stem cells and their microenvironment. Tumour Biology, 35(5), 3945–3951.
Safa, A. R. (2016). Resistance to cell death and its modulation in cancer stem cells. Critical Reviews in Oncogenesis, 21(3–4), 203–219.
Wang, H. F., et al. (2021). Cell fusion in cancer hallmarks: Current research status and future indications. Oncology Letters, 22(1), 530.
Dai, J., et al. (2020). Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduction and Targeted Therapy, 5(1), 145.
Roehlecke, C., & Schmidt, M. H. H. (2020). Tunneling nanotubes and tumor microtubes in cancer. Cancers (Basel), 12(4), 857.
Manjunath, Y., et al. (2020). Tumor-cell-macrophage fusion cells as liquid biomarkers and tumor enhancers in cancer. International Journal of Molecular Sciences, 21(5), 1872.
Jang, G., et al. (2022). Direct cell-to-cell transfer in stressed tumor microenvironment aggravates tumorigenic or metastatic potential in pancreatic cancer. NPJ Genomic Medicine, 7(1), 63.
Sharma, N., et al. (2020). Metabolic plasticity imparts erlotinib-resistance in pancreatic cancer by upregulating glucose-6-phosphate dehydrogenase. Cancer & Metabolism, 8, 19.
Biancur, D. E., & Kimmelman, A. C. (2018). The plasticity of pancreatic cancer metabolism in tumor progression and therapeutic resistance. Biochimica et Biophysica Acta - Reviews on Cancer, 1870(1), 67–75.
Boone, B. A., et al. (2015). Safety and biologic response of pre-operative autophagy inhibition in combination with gemcitabine in patients with pancreatic adenocarcinoma. Annals of Surgical Oncology, 22(13), 4402–4410.
Van Cutsem, E., et al. (2018). Phase I/II trial of pimasertib plus gemcitabine in patients with metastatic pancreatic cancer. International Journal of Cancer, 143(8), 2053–2064.
Hayes, T. K., et al. (2016). Long-term ERK inhibition in KRAS-mutant pancreatic cancer is associated with MYC degradation and senescence-like growth suppression. Cancer Cell, 29(1), 75–89.
Nishi, K., et al. (2016). Inhibition of fatty acid synthesis induces apoptosis of human pancreatic cancer cells. Anticancer Research, 36(9), 4655–4660.
Tadros, S., et al. (2017). De novo lipid synthesis facilitates gemcitabine resistance through endoplasmic reticulum stress in pancreatic cancer. Cancer Research, 77(20), 5503–5517.
Shukla, S. K., et al. (2017). MUC1 and HIF-1alpha signaling crosstalk induces anabolic glucose metabolism to impart gemcitabine resistance to pancreatic cancer. Cancer Cell, 32(1), 71-87 e7.
Biancur, D. E., et al. (2017). Compensatory metabolic networks in pancreatic cancers upon perturbation of glutamine metabolism. Nature Communications, 8, 15965.
Khalaf, K., et al. (2021). Aspects of the tumor microenvironment involved in immune resistance and drug resistance. Frontiers in Immunology, 12, 656364.
Poltavets, V., et al. (2018). The role of the extracellular matrix and its molecular and cellular regulators in cancer cell plasticity. Frontiers in Oncology, 8, 431.
Kalluri, R., & Weinberg, R. A. (2009). The basics of epithelial-mesenchymal transition. The Journal of Clinical Investigation, 119(6), 1420–1428.
Lu, W., & Kang, Y. (2019). Epithelial-mesenchymal plasticity in cancer progression and metastasis. Developmental Cell, 49(3), 361–374.
Ribatti, D., Tamma, R., & Annese, T. (2020). Epithelial-mesenchymal transition in cancer: A historical overview. Translational Oncology, 13(6), 100773.
Ruivo, C. F., et al. (2022). Extracellular vesicles from pancreatic cancer stem cells lead an intratumor communication network (EVNet) to fuel tumour progression. Gut, 71(10), 2043–2068.
Cebrian, M. J., et al. (2016). Paradoxical role of HMGB1 in pancreatic cancer: Tumor suppressor or tumor promoter? Anticancer Research, 36(9), 4381–4389.
Li, J., et al. (2020). Tumor cell-intrinsic USP22 suppresses antitumor immunity in pancreatic cancer. Cancer Immunology Research, 8(3), 282–291.
Qian, W., et al. (2021). The EGFR-HSF1 axis accelerates the tumorigenesis of pancreatic cancer. Journal of Experimental & Clinical Cancer Research, 40(1), 25.
Huang, C., Du, J., & Xie, K. (2014). FOXM1 and its oncogenic signaling in pancreatic cancer pathogenesis. Biochimica et Biophysica Acta, 1845(2), 104–116.
Zhao, J., et al. (2022). 5-fluorouracil suppresses stem cell-like properties by inhibiting p38 in pancreatic cancer cell line PANC-1. Folia Histochemica et Cytobiologica, 60(1), 55–65.
Kim, S., et al. (2015). The basic helix-loop-helix transcription factor E47 reprograms human pancreatic cancer cells to a quiescent acinar state with reduced tumorigenic potential. Pancreas, 44(5), 718–727.
Peng, L., et al. (2023). Urokinase-type plasminogen activator receptor (uPAR) cooperates with mutated KRAS in regulating cellular plasticity and gemcitabine response in pancreatic adenocarcinomas. Cancers (Basel), 15(5), 1587.
Wei, D., et al. (2023). A small molecule with big impact: MRTX1133 targets the KRASG12D mutation in pancreatic cancer. Clinical Cancer Research, 30, 1–8.
Matsubara, S., et al. (2020). Prevention of Akt phosphorylation is a key to targeting cancer stem-like cells by mTOR inhibition. Human Cell, 33(4), 1197–1203.
Peer, E., Tesanovic, S., & Aberger, F. (2019). Next-generation Hedgehog/GLI pathway inhibitors for cancer therapy. Cancers (Basel), 11(4), 538.
Nakashima, H., et al. (2006). Nuclear factor-kappaB contributes to Hedgehog signaling pathway activation through sonic Hedgehog induction in pancreatic cancer. Cancer Research, 66(14), 7041–7049.
Roca, M. S., et al. (2022). HDAC class I inhibitor domatinostat sensitizes pancreatic cancer to chemotherapy by targeting cancer stem cell compartment via FOXM1 modulation. Journal of Experimental & Clinical Cancer Research, 41(1), 83.
Capeloa, T., et al. (2022). Inhibition of mitochondrial redox signaling with MitoQ prevents metastasis of human pancreatic cancer in mice. Cancers (Basel), 14(19), 4918.
Viale, A., et al. (2014). Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature, 514(7524), 628–632.
Bao, B., et al. (2012). Metformin inhibits cell proliferation, migration and invasion by attenuating CSC function mediated by deregulating miRNAs in pancreatic cancer cells. Cancer Prevention Research (Philadelphia, Pa.), 5(3), 355–364.
Mohammed, A., et al. (2013). Antidiabetic drug metformin prevents progression of pancreatic cancer by targeting in part cancer stem cells and mTOR signaling. Translational Oncology, 6(6), 649–659.
Lonardo, E., et al. (2013). Metformin targets the metabolic achilles heel of human pancreatic cancer stem cells. PLoS One, 8(10), e76518.
Sancho, P., et al. (2015). MYC/PGC-1alpha balance determines the metabolic phenotype and plasticity of pancreatic cancer stem cells. Cell Metabolism, 22(4), 590–605.
Rausch, V., et al. (2010). Synergistic activity of sorafenib and sulforaphane abolishes pancreatic cancer stem cell characteristics. Cancer Research, 70(12), 5004–5013.
Suzuki, S., et al. (2015). JNK suppression of chemotherapeutic agents-induced ROS confers chemoresistance on pancreatic cancer stem cells. Oncotarget, 6(1), 458–470.
Shankar, S., et al. (2011). Resveratrol inhibits pancreatic cancer stem cell characteristics in human and KrasG12D transgenic mice by inhibiting pluripotency maintaining factors and epithelial-mesenchymal transition. PLoS One, 6(1), e16530.
Ben, Q., et al. (2020). A nicotine-induced positive feedback loop between HIF1A and YAP1 contributes to epithelial-to-mesenchymal transition in pancreatic ductal adenocarcinoma. Journal of Experimental & Clinical Cancer Research, 39(1), 181.
Zhang, Y., et al. (2015). Aspirin counteracts cancer stem cell features, desmoplasia and gemcitabine resistance in pancreatic cancer. Oncotarget, 6(12), 9999–10015.
Hong, S. P., et al. (2009). CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. International Journal of Cancer, 125(10), 2323–2331.
Yingling, J. M., et al. (2018). Preclinical assessment of galunisertib (LY2157299 monohydrate), a first-in-class transforming growth factor-beta receptor type I inhibitor. Oncotarget, 9(6), 6659–6677.
Melisi, D., et al. (2018). Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. British Journal of Cancer, 119(10), 1208–1214.
Zhang, G. N., et al. (2011). Combination of salinomycin and gemcitabine eliminates pancreatic cancer cells. Cancer Letters, 313(2), 137–144.
Funding
Work in the lab of Azmi AS is supported by R01CA24060701A1 and R37CA215427.
Ethics declarations
Conflict of interest
ASA received funding from Colorado Chromatography and Blackstone Therapeutics. ASA serves as a consultant for GLG and Guidepoint.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Uddin, M.H., Zhang, D., Muqbil, I. et al. Deciphering cellular plasticity in pancreatic cancer for effective treatments. Cancer Metastasis Rev 43, 393–408 (2024). https://doi.org/10.1007/s10555-023-10164-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-023-10164-5