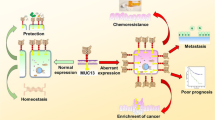
Abstract
Mucins are high-molecular-weight glycoproteins dysregulated in aggressive cancers. The role of mucins in disease progression, tumor proliferation, and chemotherapy resistance has been studied extensively. This article provides a comprehensive review of mucin’s function as a physical barrier and the implication of mucin overexpression in impeded drug delivery to solid tumors. Mucins regulate the epithelial to mesenchymal transition (EMT) of cancer cells via several canonical and non-canonical oncogenic signaling pathways. Furthermore, mucins play an extensive role in enriching and maintaining the cancer stem cell (CSC) population, thereby sustaining the self-renewing and chemoresistant cellular pool in the bulk tumor. It has recently been demonstrated that mucins regulate the metabolic reprogramming during oncogenesis and cancer progression, which account for tumor cell survival, proliferation, and drug-resistance. This review article focuses on delineating mucin’s role in oncogenic signaling and aberrant regulation of gene expressions, culminating in CSC maintenance, metabolic rewiring, and development of chemoresistance, tumor progression, and metastasis.



Similar content being viewed by others
Data availability
Not applicable
Code availability
Not applicable
Abbreviations
- ALDH:
-
aldehyde dehydrogenase
- AML:
-
acute myeloid leukemia
- CSC:
-
cancer stem cells
- EGFR:
-
epidermal growth factor receptor
- EMT:
-
epithelial-mesenchymal transition
- FAK:
-
focal adhesion kinase
- FOXO3:
-
forkhead box O-3
- hCNT1:
-
human concentrative nucleoside transporter 1
- HER2:
-
human epidermal growth factor receptor 2
- HIF-1α:
-
hypoxia-inducible factor 1, alpha
- JNK1/2:
-
c-Jun N-terminal kinases
- Lgr5:
-
leucine-rich-repeat-containing G protein-coupled receptor 5
- LIMO2:
-
LIM domain only 2
- LRP6:
-
low density lipoprotein receptor protein 6
- LSC:
-
leukemia stem cells
- MDR:
-
multiple drug resistance
- MKK7:
-
mitogen-activated protein kinase kinases 7
- mTORC1:
-
mammalian target of rapamycin complex 1
- NFkB:
-
nuclear factor-kappa B
- PTX:
-
paclitaxel
- RCC:
-
renal cell carcinoma
- SP:
-
side population
- STAT3:
-
signal transducer and activator of transcription 3
- TGFβ:
-
transforming growth factor-beta
- TGFβR I/ II:
-
transforming growth factor-beta type I/II receptor
- TIGAR:
-
TP53-induced glycolysis and apoptosis regulator
- TNBC:
-
triple negative breast cancer
- 5FU:
-
5-Fluorouracil
References
van Putten, J. P. M., & Strijbis, K. (2017). Transmembrane mucins: Signaling receptors at the intersection of inflammation and cancer. Journal of Innate Immunity, 9(3), 281–299. https://doi.org/10.1159/000453594.
Forstner, J. F. (1978). Intestinal mucins in health and disease. Digestion, 17(3), 234–263. https://doi.org/10.1159/000198115.
Kaur, S., Kumar, S., Momi, N., Sasson, A. R., & Batra, S. K. (2013). Mucins in pancreatic cancer and its microenvironment. Nature Reviews. Gastroenterology & Hepatology, 10(10), 607–620. https://doi.org/10.1038/nrgastro.2013.120.
Chaturvedi, P., Singh, A. P., & Batra, S. K. (2008). Structure, evolution, and biology of the MUC4 mucin. The FASEB Journal, 22(4), 966–981. https://doi.org/10.1096/fj.07-9673rev.
Dhanisha, S. S., Guruvayoorappan, C., Drishya, S., & Abeesh, P. (2018). Mucins: Structural diversity, biosynthesis, its role in pathogenesis and as possible therapeutic targets. Critical Reviews in Oncology/Hematology, 122, 98–122. https://doi.org/10.1016/j.critrevonc.2017.12.006.
Krishn, S. R., Ganguly, K., Kaur, S., & Batra, S. K. (2018). Ramifications of secreted mucin MUC5AC in malignant journey: A holistic view. Carcinogenesis, 39(5), 633–651. https://doi.org/10.1093/carcin/bgy019.
Ganguly, K., Rauth, S., Marimuthu, S., Kumar, S., & Batra, S. K. (2020). Unraveling mucin domains in cancer and metastasis: When protectors become predators. Cancer Metastasis Reviews, 39(3), 647–659. https://doi.org/10.1007/s10555-020-09896-5.
Lakshmanan, I., Ponnusamy, M. P., Macha, M. A., Haridas, D., Majhi, P. D., Kaur, S., Jain, M., Batra, S. K., & Ganti, A. K. (2015). Mucins in lung cancer: Diagnostic, prognostic, and therapeutic implications. Journal of Thoracic Oncology, 10(1), 19–27. https://doi.org/10.1097/jto.0000000000000404.
Pothuraju, R., Krishn, S. R., Gautam, S. K., Pai, P., Ganguly, K., Chaudhary, S., Rachagani, S., Kaur, S., & Batra, S. K. (2020). Mechanistic and functional shades of mucins and associated glycans in colon cancer. Cancers (Basel), 12(3). https://doi.org/10.3390/cancers12030649.
Gautam, S. K., Kumar, S., Dam, V., Ghersi, D., Jain, M., & Batra, S. K. (2020). MUCIN-4 (MUC4) is a novel tumor antigen in pancreatic cancer immunotherapy. Seminars in Immunology, 47, 101391. https://doi.org/10.1016/j.smim.2020.101391.
Bafna, S., Kaur, S., & Batra, S. K. (2010). Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene, 29(20), 2893–2904. https://doi.org/10.1038/onc.2010.87.
Rao, C. V., Janakiram, N. B., & Mohammed, A. (2017). Molecular pathways: Mucins and drug delivery in cancer. Clinical Cancer Research, 23(6), 1373–1378. https://doi.org/10.1158/1078-0432.Ccr-16-0862.
Moniaux, N., Escande, F., Porchet, N., Aubert, J. P., & Batra, S. K. (2001). Structural organization and classification of the human mucin genes. Frontiers in Bioscience, 6, D1192–D1206. https://doi.org/10.2741/moniaux.
Messager, M., Lefevre, J. H., Pichot-Delahaye, V., Souadka, A., Piessen, G., & Mariette, C. (2011). The impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma: A multicenter comparative study. Annals of Surgery, 254(5), 684–693; discussion 693. https://doi.org/10.1097/SLA.0b013e3182352647.
Leal, J., Smyth, H. D. C., & Ghosh, D. (2017). Physicochemical properties of mucus and their impact on transmucosal drug delivery. International Journal of Pharmaceutics, 532(1), 555–572. https://doi.org/10.1016/j.ijpharm.2017.09.018.
Bhat, P. G., Flanagan, D. R., & Donovan, M. D. (1996). Drug diffusion through cystic fibrotic mucus: Steady-state permeation, rheologic properties, and glycoprotein morphology. Journal of Pharmaceutical Sciences, 85(6), 624–630. https://doi.org/10.1021/js950381s.
Suh, H., Pillai, K., & Morris, D. L. (2017). Mucins in pancreatic cancer: Biological role, implications in carcinogenesis and applications in diagnosis and therapy. American Journal of Cancer Research, 7(6), 1372–1383.
Kalra, A. V., & Campbell, R. B. (2009). Mucin overexpression limits the effectiveness of 5-FU by reducing intracellular drug uptake and antineoplastic drug effects in pancreatic tumours. European Journal of Cancer, 45(1), 164–173. https://doi.org/10.1016/j.ejca.2008.10.008.
Shaw, L. R., Irwin, W. J., Grattan, T. J., & Conway, B. R. (2005). The influence of excipients on the diffusion of ibuprofen and paracetamol in gastric mucus. International Journal of Pharmaceutics, 290(1-2), 145–154. https://doi.org/10.1016/j.ijpharm.2004.11.028.
Sigurdsson, H. H., Kirch, J., & Lehr, C. M. (2013). Mucus as a barrier to lipophilic drugs. International Journal of Pharmaceutics, 453(1), 56–64. https://doi.org/10.1016/j.ijpharm.2013.05.040.
Jonckheere, N., Skrypek, N., & Van Seuningen, I. (2014). Mucins and tumor resistance to chemotherapeutic drugs. Biochimica et Biophysica Acta, 1846(1), 142–151. https://doi.org/10.1016/j.bbcan.2014.04.008.
Jentoft, N. (1990). Why are proteins O-glycosylated? Trends in Biochemical Sciences, 15(8), 291–294. https://doi.org/10.1016/0968-0004(90)90014-3.
van de Wiel-van Kemenade, E., Ligtenberg, M. J., de Boer, A. J., Buijs, F., Vos, H. L., Melief, C. J., Hilkens, J., & Figdor, C. G. (1993). Episialin (MUC1) inhibits cytotoxic lymphocyte-target cell interaction. Journal of Immunology, 151(2), 767–776.
Bhatia, R., Gautam, S. K., Cannon, A., Thompson, C., Hall, B. R., Aithal, A., Banerjee, K., Jain, M., Solheim, J. C., Kumar, S., & Batra, S. K. (2019). Cancer-associated mucins: role in immune modulation and metastasis. Cancer Metastasis Reviews, 38(1-2), 223–236. https://doi.org/10.1007/s10555-018-09775-0.
Hollingsworth, M. A., & Swanson, B. J. (2004). Mucins in cancer: Protection and control of the cell surface. Nature Reviews. Cancer, 4(1), 45–60. https://doi.org/10.1038/nrc1251.
Nath, S., & Mukherjee, P. (2014). MUC1: A multifaceted oncoprotein with a key role in cancer progression. Trends in Molecular Medicine, 20(6), 332–342. https://doi.org/10.1016/j.molmed.2014.02.007.
Nath, S., Daneshvar, K., Roy, L. D., Grover, P., Kidiyoor, A., Mosley, L., Sahraei, M., & Mukherjee, P. (2013). MUC1 induces drug resistance in pancreatic cancer cells via upregulation of multidrug resistance genes. Oncogenesis, 2(6), e51. https://doi.org/10.1038/oncsis.2013.16.
Pitroda, S. P., Khodarev, N. N., Beckett, M. A., Kufe, D. W., & Weichselbaum, R. R. (2009). MUC1-induced alterations in a lipid metabolic gene network predict response of human breast cancers to tamoxifen treatment. Proceedings of the National Academy of Sciences of the United States of America, 106(14), 5837–5841. https://doi.org/10.1073/pnas.0812029106.
Jin, W., Liao, X., Lv, Y., Pang, Z., Wang, Y., Li, Q., Liao, Y., Ye, Q., Chen, G., Zhao, K., & Huang, L. (2017). MUC1 induces acquired chemoresistance by upregulating ABCB1 in EGFR-dependent manner. Cell Death & Disease, 8(8), e2980. https://doi.org/10.1038/cddis.2017.378.
Ham, S. Y., Kwon, T., Bak, Y., Yu, J. H., Hong, J., Lee, S. K., Yu, D. Y., & Yoon, D. Y. (2016). Mucin 1-mediated chemoresistance in lung cancer cells. Oncogenesis, 5(1), e185. https://doi.org/10.1038/oncsis.2015.47.
Hagmann, W., Jesnowski, R., & Löhr, J. M. (2010). Interdependence of gemcitabine treatment, transporter expression, and resistance in human pancreatic carcinoma cells. Neoplasia, 12(9), 740–747. https://doi.org/10.1593/neo.10576.
Wang, W., Abbruzzese, J. L., Evans, D. B., Larry, L., Cleary, K. R., & Chiao, P. J. (1999). The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clinical Cancer Research, 5(1), 119–127.
Arlt, A., Gehrz, A., Müerköster, S., Vorndamm, J., Kruse, M. L., Fölsch, U. R., & Schäfer, H. (2003). Role of NF-kappaB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene, 22(21), 3243–3251. https://doi.org/10.1038/sj.onc.1206390.
Skrypek, N., Duchêne, B., Hebbar, M., Leteurtre, E., van Seuningen, I., & Jonckheere, N. (2013). The MUC4 mucin mediates gemcitabine resistance of human pancreatic cancer cells via the Concentrative Nucleoside Transporter family. Oncogene, 32(13), 1714–1723. https://doi.org/10.1038/onc.2012.179.
Singh, A. P., Moniaux, N., Chauhan, S. C., Meza, J. L., & Batra, S. K. (2004). Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Research, 64(2), 622–630. https://doi.org/10.1158/0008-5472.can-03-2636.
Carraway, K. L., Perez, A., Idris, N., Jepson, S., Arango, M., Komatsu, M., Haq, B., Price-Schiavi A., Zhang, J., & Carraway, C. (2002). Muc4/sialomucin complex, the intramembrane ErbB2 ligand, in cancer and epithelia: To protect and to survive. Progress in Nucleic Acid Research and Molecular Biology, 71, 149–185. https://doi.org/10.1016/s0079-6603(02)71043-x.
Chaturvedi, P., Singh, A. P., Chakraborty, S., Chauhan, S. C., Bafna, S., Meza, J. L., Singh, P. K., Hollingsworth, M. A., Mehta, P. P., & Batra, S. K. (2008). MUC4 mucin interacts with and stabilizes the HER2 oncoprotein in human pancreatic cancer cells. Cancer Research, 68(7), 2065–2070. https://doi.org/10.1158/0008-5472.Can-07-6041.
Ramsauer, V. P., Pino, V., Farooq, A., Carothers Carraway, C. A., Salas, P. J., & Carraway, K. L. (2006). Muc4-ErbB2 complex formation and signaling in polarized CACO-2 epithelial cells indicate that Muc4 acts as an unorthodox ligand for ErbB2. Molecular Biology of the Cell, 17(7), 2931–2941. https://doi.org/10.1091/mbc.e05-09-0895.
Park, S. H., Lee, J. H., Berek, J. S., & Hu, M. C. (2014). Auranofin displays anticancer activity against ovarian cancer cells through FOXO3 activation independent of p53. International Journal of Oncology, 45(4), 1691–1698. https://doi.org/10.3892/ijo.2014.2579.
Bae, J. S., Lee, J., Park, Y., Park, K., Kim, J. R., Cho, D. H., Jang, K. Y., & Park, S. H. (2017). Attenuation of MUC4 potentiates the anticancer activity of auranofin via regulation of the Her2/Akt/FOXO3 pathway in ovarian cancer cells. Oncology Reports, 38(4), 2417–2425. https://doi.org/10.3892/or.2017.5853.
Pothuraju, R., Rachagani, S., Krishn, S. R., Chaudhary, S., Nimmakayala, R. K., Siddiqui, J. A., Ganguly, K., Lakshmanan, I., Cox, J. L., Mallya, K., Kaur, S., & Batra, S. K. (2020). Molecular implications of MUC5AC-CD44 axis in colorectal cancer progression and chemoresistance. Molecular Cancer, 19(1), 37. https://doi.org/10.1186/s12943-020-01156-y.
Sheng, Y., Ng, C. P., Lourie, R., Shah, E. T., He, Y., Wong, K. Y., Seim, I., Oancea, I., Morais, C., Jeffery, P. L., Hooper, J., Gobe, G. C., & McGuckin, M. A. (2017). MUC13 overexpression in renal cell carcinoma plays a central role in tumor progression and drug resistance. International Journal of Cancer, 140(10), 2351–2363. https://doi.org/10.1002/ijc.30651.
Xu, Z., Liu, Y., Yang, Y., Wang, J., Zhang, G., Liu, Z., Fu, H., Wang, Z., Liu, H., & Xu, J. (2017). High expression of Mucin13 associates with grimmer postoperative prognosis of patients with non-metastatic clear-cell renal cell carcinoma. Oncotarget, 8(5), 7548–7558. https://doi.org/10.18632/oncotarget.13692.
Lakshmanan, I., Salfity, S., Seshacharyulu, P., Rachagani, S., Thomas, A., Das, S., Majhi, P. D., Nimmakayala, R. K., Vengoji, R., Lele, S. M., Ponnusamy, M. P., Batra, S. K., & Ganti, A. K. (2017). MUC16 regulates TSPYL5 for lung cancer cell growth and chemoresistance by suppressing p53. Clinical Cancer Research, 23(14), 3906–3917. https://doi.org/10.1158/1078-0432.Ccr-16-2530.
Das, S., Rachagani, S., Torres-Gonzalez, M. P., Lakshmanan, I., Majhi, P. D., Smith, L. M., Wagner, K., & Batra, S. K. (2015). Carboxyl-terminal domain of MUC16 imparts tumorigenic and metastatic functions through nuclear translocation of JAK2 to pancreatic cancer cells. Oncotarget, 6(8), 5772–5787. https://doi.org/10.18632/oncotarget.3308.
Boivin, M., Lane, D., Piché, A., & Rancourt, C. (2009). CA125 (MUC16) tumor antigen selectively modulates the sensitivity of ovarian cancer cells to genotoxic drug-induced apoptosis. Gynecologic Oncology, 115(3), 407–413. https://doi.org/10.1016/j.ygyno.2009.08.007.
Weissman, I. L. (2000). Stem cells: units of development, units of regeneration, and units in evolution. Cell, 100(1), 157–168. https://doi.org/10.1016/s0092-8674(00)81692-x.
Zhao, W., Ji, X., Zhang, F., Li, L., & Ma, L. (2012). Embryonic stem cell markers. Molecules, 17(6), 6196–6236. https://doi.org/10.3390/molecules17066196.
Yan, Q., Yao, D., Wei, L. L., Huang, Y., Myers, J., Zhang, L., Xin, W., Shim, J., Man, Y., Petryniak, B., Gerson, S., Lowe, J. B., & Zhou, L. (2010). O-fucose modulates Notch-controlled blood lineage commitment. The American Journal of Pathology, 176(6), 2921–2934. https://doi.org/10.2353/ajpath.2010.090702.
Seth, A., Machingo, Q. J., Fritz, A., & Shur, B. D. (2010). Core fucosylation is required for midline patterning during zebrafish development. Developmental Dynamics, 239(12), 3380–3390. https://doi.org/10.1002/dvdy.22475.
Greaves, M., & Maley, C. C. (2012). Clonal evolution in cancer. Nature, 481(7381), 306–313. https://doi.org/10.1038/nature10762.
Gupta, P. B., Onder, T. T., Jiang, G., Tao, K., Kuperwasser, C., Weinberg, R. A., & Lander, E. S. (2009). Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell, 138(4), 645–659. https://doi.org/10.1016/j.cell.2009.06.034.
Alam, M., Ahmad, R., Rajabi, H., Kharbanda, A., & Kufe, D. (2013). MUC1-C oncoprotein activates ERK → C/EBPβ signaling and induction of aldehyde dehydrogenase 1A1 in breast cancer cells. The Journal of Biological Chemistry, 288(43), 30892–30903. https://doi.org/10.1074/jbc.M113.477158.
Engelmann, K., Shen, H., & Finn, O. J. (2008). MCF7 side population cells with characteristics of cancer stem/progenitor cells express the tumor antigen MUC1. Cancer Research, 68(7), 2419–2426. https://doi.org/10.1158/0008-5472.Can-07-2249.
Stroopinsky, D., Rosenblatt, J., Ito, K., Mills, H., Yin, L., Rajabi, H., Vasir, B., Kufe, T., Luptakova, K., Arnason, J., Nardella, C., Levine, J. D., Joyce, R. M., Galinsky, I., Reiter, Y., Stone, R. M., Pandolfi, P. P., Kufe, D., & Avigan, D. (2013). MUC1 is a potential target for the treatment of acute myeloid leukemia stem cells. Cancer Research, 73(17), 5569–5579. https://doi.org/10.1158/0008-5472.Can-13-0677.
Guo, M., Luo, B., Pan, M., Li, M., Xu, H., Zhao, F., & Dou, J. (2020). Colorectal cancer stem cell vaccine with high expression of MUC1 serves as a novel prophylactic vaccine for colorectal cancer. International Immunopharmacology, 88, 106850. https://doi.org/10.1016/j.intimp.2020.106850.
Guo, M., You, C., Dong, W., Luo, B., Wu, Y., Chen, Y., Li, J., Pan, M., Li, M., Zhao, F., & Dou, J. (2020). The surface dominant antigen MUC1 is required for colorectal cancer stem cell vaccine to exert anti-tumor efficacy. Biomedicine & Pharmacotherapy, 132, 110804. https://doi.org/10.1016/j.biopha.2020.110804.
Curry, J. M., Thompson, K. J., Rao, S. G., Besmer, D. M., Murphy, A. M., Grdzelishvili, V. Z., Ahrens, W. A., McKillop, I. H., Sindram, D., Iannitti, D. A., Martinie, J. B., & Mukherjee, P. (2013). The use of a novel MUC1 antibody to identify cancer stem cells and circulating MUC1 in mice and patients with pancreatic cancer. Journal of Surgical Oncology, 107(7), 713–722. https://doi.org/10.1002/jso.23316.
Ponnusamy, M. P., Seshacharyulu, P., Vaz, A., Dey, P., & Batra, S. K. (2011). MUC4 stabilizes HER2 expression and maintains the cancer stem cell population in ovarian cancer cells. Journal of Ovarian Research, 4(1), 7. https://doi.org/10.1186/1757-2215-4-7.
Mimeault, M., Johansson, S. L., Senapati, S., Momi, N., Chakraborty, S., & Batra, S. K. (2010). MUC4 down-regulation reverses chemoresistance of pancreatic cancer stem/progenitor cells and their progenies. Cancer Letters, 295(1), 69–84. https://doi.org/10.1016/j.canlet.2010.02.015.
Jimeno, A., Feldmann, G., Suárez-Gauthier, A., Rasheed, Z., Solomon, A., Zou, G. M., Rubio-Viqueira, B., García-García, E., López-Ríos, F., Matsui, W., Maitra, A., & Hidalgo, M. (2009). A direct pancreatic cancer xenograft model as a platform for cancer stem cell therapeutic development. Molecular Cancer Therapeutics, 8(2), 310–314. https://doi.org/10.1158/1535-7163.Mct-08-0924.
Zhou, J., Wang, C. Y., Liu, T., Wu, B., Zhou, F., Xiong, J. X., Wu, H. S., Tao, J., Zhao, G., Yang, M., & Gou, S. M. (2008). Persistence of side population cells with high drug efflux capacity in pancreatic cancer. World Journal of Gastroenterology, 14(6), 925–930. https://doi.org/10.3748/wjg.14.925.
Ganguly, K., Krishn, S. R., Rachagani, S., Jahan, R., Shah, A., Nallasamy, P., Rauth, S., Atri, P., Cox, J. L., Pothuraju, R., Smith, L. M., Ayala, S., Evans, C., Ponusamy, M. P., Kumar, S., Kaur, S., & Batra, S. K. (2020). Secretory mucin 5 AC promotes neoplastic progression by augmenting KLF4-mediated pancreatic cancer cell stemness. Cancer Research. https://doi.org/10.1158/0008-5472.Can-20-1293.
Zhang, H., Yang, Y., Wang, Y., Gao, X., Wang, W., Liu, H., He, H., Liang, Y., Pan, K., Wu, H., Shi, J., Xue, H., Liang, L., Cai, Z., Fan, Y., & Zhang, Y. (2015). Relationship of tumor marker CA125 and ovarian tumor stem cells: Preliminary identification. Journal of Ovarian Research, 8, 19. https://doi.org/10.1186/s13048-015-0132-8.
Lim, S., Becker, A., Zimmer, A., Lu, J., Buettner, R., & Kirfel, J. (2013). SNAI1-mediated epithelial-mesenchymal transition confers chemoresistance and cellular plasticity by regulating genes involved in cell death and stem cell maintenance. PLoS One, 8(6), e66558. https://doi.org/10.1371/journal.pone.0066558.
Yamada, S., Fuchs, B. C., Fujii, T., Shimoyama, Y., Sugimoto, H., Nomoto, S., Takeda, S., Tanabe, K. K., Kodera, Y., & Nakao, A. (2013). Epithelial-to-mesenchymal transition predicts prognosis of pancreatic cancer. Surgery, 154(5), 946–954. https://doi.org/10.1016/j.surg.2013.05.004.
Comamala, M., Pinard, M., Thériault, C., Matte, I., Albert, A., Boivin, M., Beaudin, J., Piché, A., & Rancourt, C. (2011). Downregulation of cell surface CA125/MUC16 induces epithelial-to-mesenchymal transition and restores EGFR signalling in NIH:OVCAR3 ovarian carcinoma cells. British Journal of Cancer, 104(6), 989–999. https://doi.org/10.1038/bjc.2011.34.
Horn, G., Gaziel, A., Wreschner, D. H., Smorodinsky, N. I., & Ehrlich, M. (2009). ERK and PI3K regulate different aspects of the epithelial to mesenchymal transition of mammary tumor cells induced by truncated MUC1. Experimental Cell Research, 315(8), 1490–1504. https://doi.org/10.1016/j.yexcr.2009.02.011.
Roy, L. D., Sahraei, M., Subramani, D. B., Besmer, D., Nath, S., Tinder, T. L., Bajaj, E., Shanmugam, K., Lee, Y. Y., Hwang, S. I. L., Gendler, S. J., & Mukherjee, P. (2011). MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene, 30(12), 1449–1459. https://doi.org/10.1038/onc.2010.526.
Ponnusamy, M. P., Lakshmanan, I., Jain, M., Das, S., Chakraborty, S., Dey, P., & Batra, S. K. (2010). MUC4 mucin-induced epithelial to mesenchymal transition: A novel mechanism for metastasis of human ovarian cancer cells. Oncogene, 29(42), 5741–5754. https://doi.org/10.1038/onc.2010.309.
Lakshmanan, I., Rachagani, S., Hauke, R., Krishn, S. R., Paknikar, S., Seshacharyulu, P., Karmakar, S., Nimmakayala, R. K., Kaushik, G., Johansson, S. L., Carey, G. B., Ponnusamy, M. P., Kaur, S., Batra, S. K., & Ganti, A. K. (2016). MUC5AC interactions with integrin β4 enhances the migration of lung cancer cells through FAK signaling. Oncogene, 35(31), 4112–4121. https://doi.org/10.1038/onc.2015.478.
Hata, T., Rajabi, H., Yamamoto, M., Jin, C., Ahmad, R., Zhang, Y., Kui, L., Li, W., Yasumizu, Y., Hong, D., Miyo, M., Hiraki, M., Maeda, T., Suzuki, Y., Takahashi, H., Samur, M., & Kufe, D. (2019). Targeting MUC1-C inhibits TWIST1 signaling in triple-negative breast cancer. Molecular Cancer Therapeutics, 18(10), 1744–1754. https://doi.org/10.1158/1535-7163.Mct-19-0156.
Rajabi, H., Alam, M., Takahashi, H., Kharbanda, A., Guha, M., Ahmad, R., & Kufe, D. (2014). MUC1-C oncoprotein activates the ZEB1/miR-200c regulatory loop and epithelial-mesenchymal transition. Oncogene, 33(13), 1680–1689. https://doi.org/10.1038/onc.2013.114.
Grover, P., Nath, S., Nye, M. D., Zhou, R., Ahmad, M., & Mukherjee, P. (2018). SMAD4-independent activation of TGF-β signaling by MUC1 in a human pancreatic cancer cell line. Oncotarget, 9(6), 6897–6910. https://doi.org/10.18632/oncotarget.23966.
Rachagani, S., Macha, M. A., Ponnusamy, M. P., Haridas, D., Kaur, S., Jain, M., & Batra, S. K. (2012). MUC4 potentiates invasion and metastasis of pancreatic cancer cells through stabilization of fibroblast growth factor receptor 1. Carcinogenesis, 33(10), 1953–1964. https://doi.org/10.1093/carcin/bgs225.
Muniyan, S., Haridas, D., Chugh, S., Rachagani, S., Lakshmanan, I., Gupta, S., Seshacharyulu, P., Smith, L. M., Ponnusamy, M. P., & Batra S. K. (2016). MUC16 contributes to the metastasis of pancreatic ductal adenocarcinoma through focal adhesion mediated signaling mechanism. Genes & Cancer, 7(3–4), 110–124. https://doi.org/10.18632/genesandcancer.104.
Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: The next generation. Cell, 144(5), 646–674. https://doi.org/10.1016/j.cell.2011.02.013.
Frezza, C. (2020). Metabolism and cancer: The future is now. British Journal of Cancer, 122(2), 133–135. https://doi.org/10.1038/s41416-019-0667-3.
Jung, J. G., & Le, A. (2018). Targeting metabolic cross talk between cancer cells and cancer-associated fibroblasts. Advances in Experimental Medicine and Biology, 1063, 167–178. https://doi.org/10.1007/978-3-319-77736-8_12.
Chaika, N. V., Gebregiworgis, T., Lewallen, M. E., Purohit, V., Radhakrishnan, P., Liu, X., Zhang, B., Mehla, K., Brown, R. B., Caffrey, T., Yu, F., Johnson, K. R., Powers, R., Hollingsworth, M. A., & Singh, P. K. (2012). MUC1 mucin stabilizes and activates hypoxia-inducible factor 1 alpha to regulate metabolism in pancreatic cancer. Proceedings of the National Academy of Sciences of the United States of America, 109(34), 13787–13792. https://doi.org/10.1073/pnas.1203339109.
Kosugi, M., Ahmad, R., Alam, M., Uchida, Y., & Kufe, D. (2011). MUC1-C oncoprotein regulates glycolysis and pyruvate kinase M2 activity in cancer cells. PLoS One, 6(11), e28234. https://doi.org/10.1371/journal.pone.0028234.
Raina, D., Kharbanda, S., & Kufe, D. (2004). The MUC1 oncoprotein activates the anti-apoptotic phosphoinositide 3-kinase/Akt and Bcl-xL pathways in rat 3Y1 fibroblasts. The Journal of Biological Chemistry, 279(20), 20607–20612. https://doi.org/10.1074/jbc.M310538200.
Mehla, K., & Singh, P. K. (2014). MUC1: A novel metabolic master regulator. Biochimica et Biophysica Acta, 1845(2), 126–135. https://doi.org/10.1016/j.bbcan.2014.01.001.
Yoshida, K., & Miki, Y. (2004). Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Science, 95(11), 866–871. https://doi.org/10.1111/j.1349-7006.2004.tb02195.x.
Gunda, V., Souchek, J., Abrego, J., Shukla, S. K., Goode, G. D., Vernucci, E., Dasgupta, A., Chaika, N. V., King, R. J., Li, S., Wang, S., Yu, F., Bessho, T., Lin, C., & Singh, P. K. (2017). MUC1-mediated metabolic alterations regulate response to radiotherapy in pancreatic cancer. Clinical Cancer Research, 23(19), 5881–5891. https://doi.org/10.1158/1078-0432.Ccr-17-1151.
Chiyoda, T., Hart, P. C., Eckert, M. A., McGregor, S. M., Lastra, R. R., Hamamoto, R., Nakamura, Y., Yamada, S. D., Olopade, O. I., Lengyel, E., & Romero, I. L. (2017). Loss of BRCA1 in the cells of origin of ovarian cancer induces glycolysis: A window of opportunity for ovarian cancer Chemoprevention. Cancer Prevention Research (Philadelphia, Pa.), 10(4), 255–266. https://doi.org/10.1158/1940-6207.Capr-16-0281.
Fu, X., Tang, N., Xie, W. Q., Mao, L., & Qiu, Y. D. (2020). MUC1 promotes glycolysis through inhibiting BRCA1 expression in pancreatic cancer. Chinese Journal of Natural Medicines, 18(3), 178–185. https://doi.org/10.1016/s1875-5364(20)30019-4.
Leng, Y., Cao, C., Ren, J., Huang, L., Chen, D., Ito, M., & Kufe, D. (2007). Nuclear import of the MUC1-C oncoprotein is mediated by nucleoporin Nup62. The Journal of Biological Chemistry, 282(27), 19321–19330. https://doi.org/10.1074/jbc.M703222200.
Raina, D., Ahmad, R., Rajabi, H., Panchamoorthy, G., Kharbanda, S., & Kufe, D. (2012). Targeting cysteine-mediated dimerization of the MUC1-C oncoprotein in human cancer cells. International Journal of Oncology, 40(5), 1643–1649. https://doi.org/10.3892/ijo.2011.1308.
Raina, D., Kosugi, M., Ahmad, R., Panchamoorthy, G., Rajabi, H., Alam, M., Shimamura, T., Shapiro, G. I., Supko, J., Kharbanda, S., & Kufe, D. (2011). Dependence on the MUC1-C oncoprotein in non-small cell lung cancer cells. Molecular Cancer Therapeutics, 10(5), 806–816. https://doi.org/10.1158/1535-7163.Mct-10-1050.
GongSun, X., Zhao, Y., Jiang, B., Xin, Z., Shi, M., Song, L., Qin, Q. M., Wang, Q., & Liu, X. Y. (2019). Inhibition of MUC1-C regulates metabolism by AKT pathway in esophageal squamous cell carcinoma. Journal of Cellular Physiology, 234(7), 12019–12028. https://doi.org/10.1002/jcp.27863.
Kumari, S., Khan, S., Gupta, S. C., Kashyap, V. K., Yallapu, M. M., Chauhan, S. C., & Jaggi, M. (2018). MUC13 contributes to rewiring of glucose metabolism in pancreatic cancer. Oncogenesis, 7(2), 19. https://doi.org/10.1038/s41389-018-0031-0.
Haridas, D., Chakraborty, S., Ponnusamy, M. P., Lakshmanan, I., Rachagani, S., Cruz, E., Kumar, S., Das, S., Lele, S. M., Anderson, J. M., Wittel, U. A., Hollingsworth, M. A., & Batra, S. K. (2011). Pathobiological implications of MUC16 expression in pancreatic cancer. PLoS One, 6(10), e26839. https://doi.org/10.1371/journal.pone.0026839.
Shukla, S. K., Gunda, V., Abrego, J., Haridas, D., Mishra, A., Souchek, J., Chaika, N. V., Yu, F., Sasson, A. R., Lazenby, A. J., Batra, S. K., & Singh, P. K. (2015). MUC16-mediated activation of mTOR and c-Myc reprograms pancreatic cancer metabolism. Oncotarget, 6(22), 19118–19131. https://doi.org/10.18632/oncotarget.4078.
Shimobayashi, M., & Hall, M. N. (2014). Making new contacts: The mTOR network in metabolism and signalling crosstalk. Nature Reviews. Molecular Cell Biology, 15(3), 155–162. https://doi.org/10.1038/nrm3757.
Düvel, K., Yecies, J. L., Menon, S., Raman, P., Lipovsky, A. I., Souza, A. L., Triantafellow, E., Ma, Q., Gorski, R., Cleaver, S., Vander Heiden, M. G., MacKeigan, J. P., Finan, P. M., Clish, C. B., Murphy, L. O., & Manning, B. D. (2010). Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Molecular Cell, 39(2), 171–183. https://doi.org/10.1016/j.molcel.2010.06.022.
Nomura, D. K., & Cravatt, B. F. (2013). Lipid metabolism in cancer. Biochimica et Biophysica Acta, 1831(10), 1497–1498. https://doi.org/10.1016/j.bbalip.2013.08.001.
Mashima, T., Seimiya, H., & Tsuruo, T. (2009). De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. British Journal of Cancer, 100(9), 1369–1372. https://doi.org/10.1038/sj.bjc.6605007.
Liu, Y. (2006). Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer and Prostatic Diseases, 9(3), 230–234. https://doi.org/10.1038/sj.pcan.4500879.
Mukherjee, A., Wu, J., Barbour, S., & Fang, X. (2012). Lysophosphatidic acid activates lipogenic pathways and de novo lipid synthesis in ovarian cancer cells. The Journal of Biological Chemistry, 287(30), 24990–25000. https://doi.org/10.1074/jbc.M112.340083.
Martel, P. M., Bingham, C. M., McGraw, C. J., Baker, C. L., Morganelli, P. M., Meng, M. L., Armstrong, J. M., Moncur, J. T., & Kinlaw, W. B., (2006). S14 protein in breast cancer cells: Direct evidence of regulation by SREBP-1c, superinduction with progestin, and effects on cell growth. Experimental Cell Research, 312(3), 278–288. https://doi.org/10.1016/j.yexcr.2005.10.022.
Migita, T., Narita, T., Nomura, K., Miyagi, E., Inazuka, F., Matsuura, M., Ushijima, M., Mashima, T., Seimiya, H., Satoh, Y., Okumura, S., Nakagawa, K., & Ishikawa, Y. (2008). ATP citrate lyase: Activation and therapeutic implications in non-small cell lung cancer. Cancer Research, 68(20), 8547–8554. https://doi.org/10.1158/0008-5472.Can-08-1235.
Menendez, J. A., & Lupu, R. (2007). Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nature Reviews. Cancer, 7(10), 763–777. https://doi.org/10.1038/nrc2222.
Furuta, E., Pai, S. K., Zhan, R., Bandyopadhyay, S., Watabe, M., Mo, Y. Y., Hirota, S., Hosobe, S., Tsukada, T., Miura, K., Kamada, S., Saito, K., Iiizumi, M., Liu, W., Ericsson, J., & Watabe, K. (2008). Fatty acid synthase gene is upregulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Research, 68(4), 1003–1011. https://doi.org/10.1158/0008-5472.Can-07-2489.
Taylor-Papadimitriou, J., Burchell, J. M., Graham, R., & Beatson, R. (2018). Latest developments in MUC1 immunotherapy. Biochemical Society Transactions, 46(3), 659–668. https://doi.org/10.1042/bst20170400.
Panchamoorthy, G., Jin, C., Raina, D., Bharti, A., Yamamoto, M., Adeebge, D., Zhao, Q., Bronson, R., Jiang, S., Li, L., Suzuki, Y., Tagde, A., Ghoroghchian, P. P., Wong, K. K., Kharbanda, S., & Kufe, D. (2018). Targeting the human MUC1-C oncoprotein with an antibody-drug conjugate. JCI Insight, 3(12). https://doi.org/10.1172/jci.insight.99880.
Liberelle, M., Magnez, R., Thuru, X., Bencheikh, Y., Ravez, S., Quenon, C., Drucbert, A. S., Foulon, C., Melnyk, P., van Seuningen, I., & Lebègue, N. (2019). MUC4-ErbB2 oncogenic complex: Binding studies using microscale thermophoresis. Scientific Reports, 9(1), 16678. https://doi.org/10.1038/s41598-019-53099-0.
Das, S., & Batra, S. K. (2015). Understanding the unique attributes of MUC16 (CA125): Potential implications in targeted therapy. Cancer Research, 75(22), 4669–4674. https://doi.org/10.1158/0008-5472.Can-15-1050.
Aithal, A., Rauth, S., Kshirsagar, P., Shah, A., Lakshmanan, I., Junker, W. M., Jain, M., Ponnusamy, M. P., & Batra, S. K. (2018). MUC16 as a novel target for cancer therapy. Expert Opinion on Therapeutic Targets, 22(8), 675–686. https://doi.org/10.1080/14728222.2018.1498845.
Patel, S. P., Bristol, A., Saric, O., Wang, X. P., Dubeykovskiy, A., Arlen, P. M., & Morse, M. A. (2013). Anti-tumor activity of a novel monoclonal antibody, NPC-1C, optimized for recognition of tumor antigen MUC5AC variant in preclinical models. Cancer Immunology, Immunotherapy, 62(6), 1011–1019. https://doi.org/10.1007/s00262-013-1420-z.
Acknowledgements
We thank Kavita Mallya and Corinn E Grabow for their support.
Funding
The authors in this article were supported primarily by the following grants from the National Institutes of Health P01 CA217798, R01 CA210637, R01 CA183459, R01 CA195586, R01 CA201444, R01 CA228524, F99 CA234962, U01 CA200466, and U01 CA210240, and the Nebraska Department of Health and Human Services LB595.
Author information
Authors and Affiliations
Contributions
SM, SR, CZ, and KG, collected the related literature and drafted the manuscript. SM, SR, CZ, KG, IL, MPP, and SKB participated in the manuscript’s draft. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Conflict of interest/Competing interests
SKB is one of the co-founders of Sanguine Diagnostics and Therapeutics, Inc. The other authors disclosed no potential conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marimuthu, S., Rauth, S., Ganguly, K. et al. Mucins reprogram stemness, metabolism and promote chemoresistance during cancer progression. Cancer Metastasis Rev 40, 575–588 (2021). https://doi.org/10.1007/s10555-021-09959-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-021-09959-1