Abstract
Purpose
In the Women’s Health initiative (WHI) randomized clinical trial, conjugated equine estrogen (CEE)-alone significantly reduced breast cancer incidence (P = 0.005). As cohort studies had opposite findings, other randomized clinical trials were identified to conduct a meta-analysis of estrogen-alone influence on breast cancer incidence.
Methods
We conducted literature searches on randomized trials and: estrogen, hormone therapy, and breast cancer, and searches from a prior meta-analysis and reviews. In the meta-analysis, for trials with published relative risks (RR) and 95% confidence intervals (CI), each log-RR was multiplied by weight = 1/V, where V = variance of the log-RR, and V was derived from the corresponding 95% CI. For smaller trials with only breast cancer numbers, the corresponding log-RR = (O – E)/weight, where O is the observed case number in the oestrogen-alone group and E the corresponding expected case number, E = nP.
Results
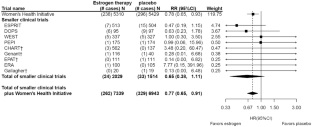
Findings from 10 randomized trials included 14,282 participants and 591 incident breast cancers. In 9 smaller trials, with 1.2% (24 of 2029) vs 2.2% (33 of 1514) randomized to estrogen-alone vs placebo (open label, one trial) (RR 0.65 95% CI 0.38–1.11, P = 0.12). For 5 trials evaluating estradiol formulations, RR = 0.63 95% CI 0.34–1.16, P = 0.15. Combining the 10 trials, 3.6% (262 of 7339) vs 4.7% (329 of 6943) randomized to estrogen-alone vs placebo (overall RR 0.77 95% CI 0.65–0.91, P = 0.002).
Conclusion
The totality of randomized clinical trial evidence supports a conclusion that estrogen-alone use significantly reduces breast cancer incidence.

Similar content being viewed by others
Data availability
All the data are available from the sources cited in the manuscript.
References
Collaborative Group on Hormonal Factors in Breast C (2019) Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet 394(10204):1159–1168
Chlebowski RT, Anderson GL, Aragaki AK et al (2020) Association of Menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA 324(4):369–380
The Writing Group for the PEPI Trial (1995) Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA 273(3):199–208
Herrington DM, Reboussin DM, Brosnihan KB et al (2000) Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med 343(8):522–529
Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI (2001) A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med 345(17):1243–1249
Schierbeck LL, Rejnmark L, Tofteng CL et al (2012) Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ 345:e6409
Cherry N, McNamee R, Heagerty A, Kitchener H, Hannaford P (2014) Long-term safety of unopposed estrogen used by women surviving myocardial infarction: 14-year follow-up of the ESPRIT randomised controlled trial. BJOG 121(6):700–705; discussion 5
Chlebowski RT, Rohan TE, Manson JE et al (2015) Breast cancer after use of estrogen plus progestin and estrogen alone: analyses of data from 2 Women’s Health Initiative randomized clinical trials. JAMA Oncol 1(3):296–305
Manson JE, Chlebowski RT, Stefanick ML et al (2013) Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA 310(13):1353–1368
Gartlehner G, Patel SV, Feltner C et al (2017) Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: evidence report and systematic review for the US preventive services task force. JAMA 318(22):2234–2249
Hemminki E, McPherson K (1997) Impact of postmenopausal hormone therapy on cardiovascular events and cancer: pooled data from clinical trials. BMJ 315(7101):149–153
Hodis HN, Mack WJ, Lobo RA et al (2001) Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 135(11): 939–953
Speroff L, Rowan J, Symons J, Genant H, Wilborn W (1996) The comparative effect on bone density, endometrium, and lipids of continuous hormones as replacement therapy (CHART study) A randomized controlled trial. JAMA 276(17):1397–1403
Genant HK, Baylink DJ, Gallagher JC, Harris ST, Steiger P, Herber M (1990) Effect of estrone sulfate on postmenopausal bone loss. Obstet Gynecol 76(4):579–584
Gallagher JC, Kable WT, Goldgar D (1991) Effect of progestin therapy on cortical and trabecular bone: comparison with estrogen. Am J Med 90(2):171–178
Waters DD, Alderman EL, Hsia J et al (2002) Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: a randomized controlled trial. JAMA 288(19):2432–2440
Greenspan SL, Resnick NM, Parker RA (2005) The effect of hormone replacement on physical performance in community-dwelling elderly women. Am J Med 118(11):1232–1239
Stefanick ML, Anderson GL, Margolis KL et al (2006) Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy. JAMA 295(14):1647–1657
Bradburn MJ, Deeks JJ, Berlin JA, Russell LA (2007) Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med 26(1):53–77
Sweeting MJ, Sutton AJ, Lambert PC (2004) What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 23(9):1351–1375
Hirji KF, Mehta CR, Patel NR (1987) Computing distributions for exact logistic regression. J Am Stat Assoc 82:1110–1117
Chlebowski RT, Anderson G, Manson JE et al (2010) Estrogen alone in postmenopausal women and breast cancer detection by means of mammography and breast biopsy. J Clin Oncol 28(16):2690–2697
Anderson GL, Chlebowski RT, Aragaki AK et al (2012) Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol 13(5):476–486
Dauphine C, Moazzez A, Neal JC, Chlebowski RT, Ozao-Choy J (2020) Single hormone receptor-positive breast cancers have distinct characteristics and survival. Ann Surg Oncol 27(12):4687–4694
Fisher B, Costantino JP, Wickerham DL et al (2005) Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 97(22):1652–1662
Powles TJ, Ashley S, Tidy A, Smith IE, Dowsett M (2007) Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst 99(4):283–290
Veronesi U, Maisonneuve P, Rotmensz N et al (2007) Tamoxifen for the prevention of breast cancer: late results of the Italian Randomized Tamoxifen Prevention Trial among women with hysterectomy. J Natl Cancer Inst 99(9):727–737
Cuzick J, Sestak I, Bonanni B et al (2013) Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet 381(9880):1827–1834
Cuzick J, Sestak I, Cawthorn S et al (2015) Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol 16(1):67–75
Nelson HD, Fu R, Zakher B, Pappas M, McDonagh M (2019) Medication use for the risk reduction of primary breast cancer in women: updated evidence report and systematic review for the US preventive services task force. JAMA 322(9):868–886
Chlebowski RT, Aragaki AK (2023) The Women’s Health Initiative randomized trials of menopausal hormone therapy and breast cancer: findings in context. Menopause 30(4):454–461
Santen RJ, Stuenkel CA, Yue W (2022) Mechanistic effects of estrogens on breast cancer. Cancer J 28(3):224–240
Jordan VC, Ford LG (2011) Paradoxical clinical effect of estrogen on breast cancer risk: a “new” biology of estrogen-induced apoptosis. Cancer Prev Res (Phila) 4(5):633–637
Abderrahman B, Jordan VC (2022) Estrogen for the treatment and prevention of breast cancer: a tale of 2 Karnofsky lectures. Cancer J 28(3):163–168
Santen RJ, Yue W (2019) Cause or prevention of breast cancer with estrogens: analysis from tumor biologic data, growth kinetic model and Women’s Health Initiative study. Climacteric 22(1):3–12
Ellis MJ, Gao F, Dehdashti F et al (2009) Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. JAMA 302(7):774–780
Beral V, Reeves G, Bull D, Green J, Million Women Study C (2011) Breast cancer risk in relation to the interval between menopause and starting hormone therapy. J Natl Cancer Inst 103(4):296–305
Beral V, Peto R, Pirie K, Reeves G (2019) Menopausal hormone therapy and 20-year breast cancer mortality. Lancet 394(10204):1139
Manson JE, Aragaki AK, Rossouw JE et al (2017) Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA 318(10):927–938
Prentice RL, Aragaki AK, Chlebowski RT et al (2021) Randomized trial evaluation of the benefits and risks of menopausal hormone therapy among women 50–59 years of age. Am J Epidemiol 190(3):365–375
Goss PE, Ingle JN, Ales-Martinez JE et al (2011) Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 364(25):2381–2391
Acknowledgements
We acknowledge the commitment of the WHI investigators, staff, and the trial participants. Program Office: Jacques Roscoe, Shari Ludlum, Dale Burden, Joan McGowan, Leslie Ford, and Nancy Geller (National Heart, Lung, and Blood Institute, Bethesda, MD) Clinical Coordinating Center: Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kopperberg (Fred Hutchinson Cancer Research Center, Seattle, WA) Investigators and Academic Centers: JoAnn E. Manson (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA); Barbara V. Howard (MedStar Health Research Institute/Howard University, Washington, DC); Marcia L. Stefanick (Stanford Prevention Research Center, Stanford, CA); Rebecca Jackson (The Ohio State University, Columbus, OH); Cynthia A. Thompson (University of Arizona, Tucson/Phoenix, AZ); Jean Wactawski-Wende (University at Buffalo, Buffalo, NY); Marian Limacher (University of Florida, Gainesville/Jacksonville, FL); Robert Wallace (University of Iowa, Iowa City/Davenport, IA); Lewis Kuller (University of Pittsburgh, Pittsburgh, PA); Rowan T Chlebowski (The Lundquist Institute, Torrance, CA; and Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, NC).
A full list of all the investigators who have contributed to WHI science can be retrieved at: https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator %20Long%20List.pdf.
Funding
The development of this paper is partially supported by the National Cancer Institute grants R01 CA119171 and R01 CA10921. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C.
Author information
Authors and Affiliations
Contributions
Rowan Chlebowski and Aaron Aragaki contributed to the study concept and design. Aaron Aragaki performed data collection. Both Rowan Chlebowski and Aaron Aragaki contributed to the first draft of the manuscript and provided the statistical analysis. All authors provided critical manuscript review and revision for important intellectual content. Rowan Chlebowski, Karen Johnson, Jean Wactawski-Wende, Dorothy Lane, and JoAnn Manson obtained funding. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable, as all data used were from published sources.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chlebowski, R.T., Aragaki, A.K., Pan, K. et al. Randomized trials of estrogen-alone and breast cancer incidence: a meta-analysis. Breast Cancer Res Treat (2024). https://doi.org/10.1007/s10549-024-07307-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10549-024-07307-9




